|
|
|
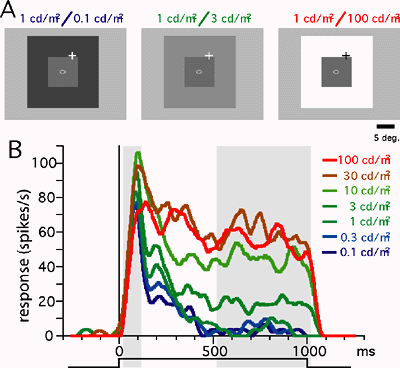
DEPARTMENT OF INFORMATION PHYSIOLOGYDivision of Sensory and Cognitive InformationThe main purpose of this division is to study the neural mechanisms of visual perception. The human visual system is a complicated parallel and distributed system where several neural structures play different roles, but are still able to generate a unified and integrated precept of the outer world. This system also has sophisticated mechanisms that enable reconstruction of three-dimensional structures from two-dimensional retinal images. To understand the neural substrates of these abilities in our visual system, we are recording neuronal activities from the primary visual cortex and extrastriate visual areas. We are analyzing the stimulus selectivity of neurons to determine the representation of various kinds of visual features, such as color, motion, shape and depth. We are also studying the dynamics of visual information processing in the cortex by analyzing the temporal pattern of neural activities. In addition, to explore the ways in which various visual features contribute to visual perception, psychophysical experiments are conducted in this laboratory.
Staff
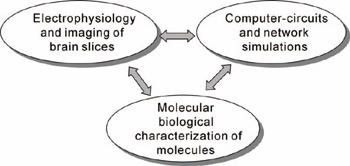
Division of Neural SignalingOur main interest lies in elucidation of the mechanism of transduction and integration of neural information in the nervous system. More specifically, we are trying to understand the basic properties of neural information processing between neurons or among a group of neurons constituting a local network. We are also interested in the pathophysiological mechanism how a single gene mutation leads to a symptom (such as ataxia and epilepsy), particularly in Ca2+ channel mutant mice. Additionally, we have recently started to make a computational approach, incorporating computer-based neurons into brain slice measurements, together with computational simulation of network functions (Fig.1). The following are currently ongoing major projects. (1) Molecular biological analysis of voltage-gated calcium channels and functional studies of their-associated neurological disorders. Recently, mutations of the voltage-gated calcium channels were found to be associated with neurological disorders of human and mice, which include cerebellar ataxia and some forms of seizure disorders. We study the relation how a single mutation causes neurological manifestations, mainly using brain slice preparations (Fig 2). (2)Integration of sensory inputs in the thalamus. Recent studies revealed the mechanism of processing the sensory information at the peripheral nerves and the spinal cord, little is known about the operational mechanisms in the thalamus. We have identified PLCb4, which is abundantly expressed in the thalamus, as a key molecule for inflammatory pains. Currently we analyze a model mice lacking the PLCb4, gene, and try to understand the role of thalamic neurons in the sensory processing system. (3) Interaction between heterologous synapses. The synapses, which mediate information between neurons, can be classified into two types; excitatory and inhibitory. Although, in principle, the direction of information in the synapse is uni-directional, recent studies showed that synaptic transmission can be retrograde or heterosynaptic. We found that the excitatory neurotransmitters released from climbing fiber terminals (projecting to cerebellar Purkinje cells from the inferior olive in the brain stem) suppress the inhibitory synaptic transmission from basket cells to Purkinje cells (dis-inhibition). Combination of excitatory input and suppression of inhibitory input seems a refined mechanism to enhance the cerebellar output. We are trying to elucidate the molecular mechanism and physiological significance of the interesting phenomenon.
Staff
Division of Neurobiology and Behavioral GeneticsDivision of Learning and Memory Research |
|
|
|
| Copyright(C) 2005 NIPS (www-admin@nips.ac.jp) | |