|
Diverse types of membrane proteins act in concert to form excitabilities in neurons and muscle cells. To establish membrane excitabilities in the context of cell physiology and embryogenesis, single cells need to integrate expression of ion channels in a manner specific to subcellular compartments and developmental stages. We are studying on function and regulation of membrane proteins, mainly ion channels, by focusing on the following biological events.
1. Physiological role of membrane potential and novel voltage-sensing proteins
Voltage sensors have long been thought as traits unique to voltage-gated ion channels that underlie membrane excitabilities. We have recently identified a novel protein that contains voltage sensor similar to ion channel and phosphatase. This protein shows voltage-dependent tuning of phosphatase activity based on the operation of voltage sensor. This indicates that voltage sensing is not confined to ion channels but could be more widespread than previously realized. We are currently asking how voltage sensor operation is coupled to phosphatase activity, and what kind of biological context this protein is involved.
2. How are neuron-subtype-specific functional traits established during development?
There are ten genes coding alpha-subunits of voltage-gated sodium channels (Nav channels) in mammals and four genes are expressed in CNS neurons. However, the same Nav channel gene, for example, Nav1.6, underlies diverse types of sodium currents which distribute on different compartments of neurons. Such diversity of Nav channel function may depend on interacting proteins or cellular environments. Spinal neurons consist of multiple populations with distinct functional properties including excitability, transmitter phenotype and innervation patterns. We aim to reveal how cell-specificity and diversity of neuronal functions are acquired in CNS development by integrating methods of electrophysiology, cell biology and molecular biology.
3. How do membrane excitabilities play role in development?
Embryos at distinct developmental stages express different sets of ion channel molecules. In particular, Ca channel provide Ca2+ influx which plays important roles in several steps of development, including fertilization, morphogenesis, cell fate determination, cell migration, neurite extention and cell survival. However, little is known how membrane excitability leads to dynamic cellular events during development. We are trying to understand roles of electrical activities during development by modulating membrane excitabilities by using molecular biological tools in developing embryos. We also explore roles of novel membrane proteins related to membrane excitabilities.
4. How is vertebrate locomotion system originated from the ancestral chordate?
Embryos of ascidian and appendiculum develop very rapidly into swimming tadpole-like larva and show conserved organization and behavior similar to primitive vertebrates. These larvae have a nervous system with much smaller number of neurons than in the vertebrate. Locomotion system in these lower chordates is being studied at both levels of cellular organization and electrophysiology as compared with that in vertebrates.
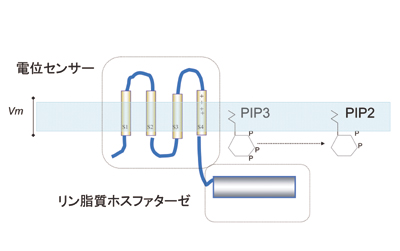
Novel voltage-sensing protein
We have recently discovered a novel protein that contains channel-like voltage sensor and phosphatase. Phosphatase activity is tuned by the operation of its intrinsic voltage sensor in response to change of membrane potential. This suggests a novel signaling pathway coupled with membrane potential change without requiring ionic flow.
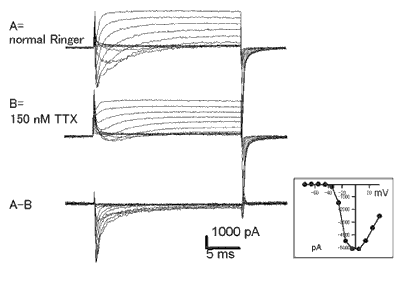
Patch clamp recording of somal sodium currents from Purkinje neurons in slice preparation.
Voltage-gated sodium currents were recorded in outside-out patch mode. TTX-sensitive component was obtained as the sodium current component after subtraction of trace before and after bath application of TTX.
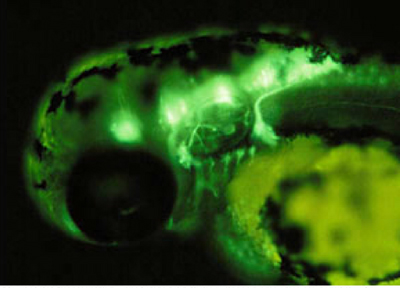
Studies with zebrafish as a model system to understand molecular mechanisms underlying development of neuronal wiring and neurophysiology of locomotion.
In the transgenic zebrafish, remove neurons are easily identified by fluorescence of GFP in live animals.
Staff
 |
Professor (concurrent, NIPS):
OKAMURA, Yasushi, MD, PhD
1985 Graduated from University of Tokyo, Faculty of Medicine. 1989 Completed the doctoral course in Medical Science, University of Tokyo. 1995 Senior Researcher, National Institute of Bioscience and Human-Technology. 2001 Professor, NIPS.
Speciality: Developmental Neurobiology, Ion channel biophysics |
 |
Associate Professor (concurrent, NIPS):
HIGASHIJIMA, Shin-ichi, PhD
1989 Graduated from University of Tokyo, Faculty of Science. 1994 Completed the doctoral course in Science, University of Tokyo. 1994 Research Associate, National Institute for Basic Biology. 1996 PREST Researcher. 1998 Research Scientist, State University of New York at Stony Brook. 2003 Associate Professor, NIPS.
Speciality: Developmental Neurobiology, Neurophysiology |
 |
Assistant Professor (NIPS):
KUKITA, Fumio, PhD
1971 Graduated from the University of Tokyo, Faculty of Science. 1976 Completed the doctoral course in physics at the University of Tokyo, 1977 Research Associate, NIPS.
Speciality: Biophysics and Molecular Physiology |
 |
Assistant Professor (NIPS):
IWASAKI, Hirohide, PhD
1994 Graduated from Tokyo Institute of Technology, Department of Bioscience and Biotechno074-075-1102logy. 2001 Completed the doctoral course in Medical Science, University of Tokyo. 2001 Special Postdoctral Fellow,The Institute of Physical and Chemical Research(RIKEN). 2002 Research Associate,NIPS.
Speciality: Developmental Neurobiology |
 |
Research Fellow (NIPS):
KIMURA, Yukiko, PhD
1999 Graduated from Saitama University. 2004 Completed the doctoral course in biological sciences, the University of Tokyo. 2004 Research fellow, NIPS.
Speciality: Developmental biology |
 |
JSPS Research Fellow:
NISHINO, Atsuo, PhD
1997 Graduated from University of Tokyo, Faculty of Science. 2001 Left the doctor course in Graduate School of Science, Kyoto University. 2001 Research Associate, Graduate School of Frontier Sciences, University of Tokyo. 2004 JSPS Postdoctral Research Fellow.
Speciality: Invertebrate Zoology |
 |
JSPS Research Fellow:
NAKAYAMA, Kiyomi, PhD
1995 Graduated from Tokyo Medical and Dental University. 2003 Completed the doctoral course in Medical Science, University of Tsukuba. 2004 Research Fellow, JSPS.
Speciality: Neurophysiology |
 |
JSPS Research Fellow:
MURATA, Yoshimichi, PhD
1996 Graduated from Meiji Pharmaceutical University, Faculty of Pharmacy. 2002 Completed the doctoral course in in Medical Science at Tokyo Medical and Dental University. 2002 Research fellow, NIPS, 2004 JSPS Research fellow.
Speciality: Ion channel biophysics |
A novel methodology, when it is very informative, gives an aid to the opening of a novel scientific field. For example, MRI originally born from NMR in chemistry and primarily developed for diogonoes has outgrown to cover almost all medical sciences. We call such a productive innovation, emerged from an old regime but creative to a new field, as a strategic methodology. Integration of biosciences might bring about such a difficulty that a simple sum of constituent disciplines never makes a good start. Fusion of different disciplines can be encouraged by novel breakthroughs in methodology. The expected are new methods for three-dimensional structural analysis of single biological molecules and the in situ functional observation of complex biological systems.
This laboratory works on above methodological themes by relying on the technical breakthrough of imaging methods such as electron microscopy.
(1) Development and application of electron-phase microscopy: Different kinds of phase observation schemes have been developed including the novel optical principle for the reconstruction of complex wavefunctions. They are expected to enhance the contrast which is inherently very poor in electron microscopy of biological samples. Applications are:
- direct visualization and determination of primary structures (sequences) of a single DNA molecule,
- structural and functional analyses of membrane proteins with the aid of single particle analysis and systematic two-dimensional crystallization.
(2) Biological transports: Transcellular and paracellurar mechanisms for transport of water, electrolytes, and substrates are investigated by laying much emphasis on molecular mechanism of exocytosis and energy supply for transport in the exocrine glands.
(3) Aging process is, at least partially, regulated by the intracellular transport of neurotransmitter receptors and hormone receptors. We investigate the mechanism of receptor traffic especially via posttranslational modifications. Toward this goal, we have identified several novel modification enzymes and we are developing new technologies to visualize posttranslational modifications in vivo.
(4) Sorting in the endocytic pathway: The endocytic pathway functions as a sorting station for molecules that are destined either for lysosomes (a degrative pathway) or for recycling pathways, thereby determining the fate of endomembrane molecules. The physiological roles and the mechanisms of sorting in the endocytic pathway are investigated.
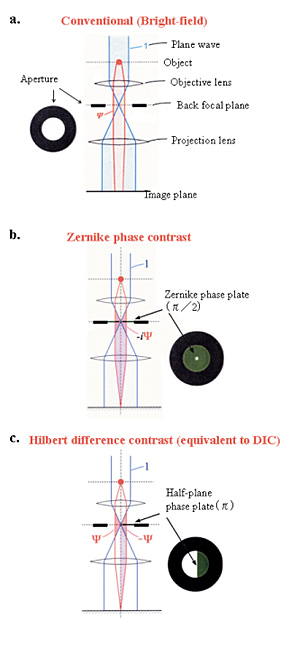 |
Fig. 1 Three kinds of schemes for electron-phase microscopy.
a. Conventional (bright-field) method to enhance the image contrast at the expense of a dehancement of the spatial resolution by defocusing.
b. Zernike phase contrast method to enhance the image contrast under the just focus condition by inserting a Zernike phase plate to the objective back-focal plane.
c. Hilbert differential method to obtain phase contrast images similar to light-microscopic DIC (Differential-Interference-Contrast) images by inserting a half-plane p phase plate to the objective back-focal plane.
|
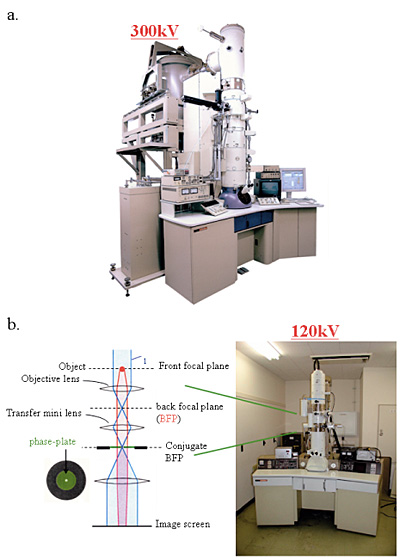
Fig. 2 Two kinds of electron-phase microscope models.
a. 300kV analytical cryo-electron microscope (equipped with a FEG, a He-stage and a w-filter) equipping phase plates at the back-focal plane.
b. 120kV TEM particularized to the phase contrast observation by furnishing a lens system immediately below the objective and fasillitating heating and precise positioning of phase plates.
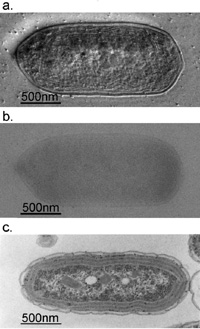 |
Fig. 3 Comparison of 300kV and 100kV TEM images for ice-embedded and plastic-embedded cyanobacterial cells.
a. A 300kV Hilbert differential image for an ice-embedded cyanobacterial whole cell, which holds a resolution sufficient for the identification of subcellular structures down to 2nm.
b. A 300kV conventional image shot for just the same sample as shown in a., of which low contrast makes it difficult to identify subcellular structures.
c. A 100kV conventional image for a plastic-embedded and thin-sectioned cyanobacterial cell, which was prepared with a chemical fixation, dehydration and a heavy metal staining. Due to the harsh and lengthy chemical treatments, subcellular structures are heavily damaged making their morphological preservation hard.
|
Staff
 |
Professor (concurrent, NIPS):
NAGAYAMA, Kuniaki, PhD
1968 Graduated from University of Tokyo. 1973 Completed the doctoral course in Science, University of Tokyo. 1974 Research Associate, University of Tokyo. 1984 Director, Biometrology Lab, JEOL Ltd. 1990 Project Leader, Nagayama Protein Array Project, ERATO, JRDC. 1993 Professor, The University of Tokyo. 1997 Professor, NIPS. 2001 Professor, Okazaki Institute for Integrative Bioscience (OIB).
Speciality: Biophysics, Electron Microscopy |
 |
Associate Professor (NIPS):
MURAKAMI, Masataka, MB, M.D.
1976 Graduated from Kyoto Prefectural University of Medicine. 1976 Research Associate, Osaka Medical College. 1981 Doctor of Medicine in Physiology of Osaka Medical College. 1983 Postdoctorial Fellow, Department of Physiology, University of Sydney. 1985 Associate Professor, NIPS. 2003 Associate Professor, OIB (Seconded from NIPS).
Speciality: Physiology of exocrine glands, Energy metabolism and transport of electrolyte and water, Paracellular Transport. |
 |
Associate Professor:
SETOU, Mitsutoshi, MD, PhD
1994 Graduated from University of Tokyo, School of Medicine. 1998 Research Associate, University of Tokyo, School of Medicine. 2001 Doctor of Medicine in Cell biology and anatomy of University of Tokyo, School of Medicine. 2002 Principal Investigator of PRESTO, Japan Science and Technology Corporation. 2003 Associate Professor.
Speciality: Cell Biology and Anatomy, Intracellular transport, Receptor dynamics, Aging |
 |
Assistant Professor:
OHASHI, Masato, PhD
1986 Graduated from Kyoto University, Faculty of Science. 1992 Completed the doctoral course in Science, Kyoto University. 1992 Postdoctoral Fellow, Department of Neurobiology, University of Heidelberg. 1996 Assistant Professor, NIPS. 2003 Assistant Professor, OIB.
Speciality: Cell Biology |
 |
Postdoctoral Fellow:
DANEV, Radostin, PhD
1997 Graduated from Faculty of Physics, Sofia University, Sofia, Bulgaria. 2001 Completed the doctoral course in Science, The Graduate University for Advanced Studies, NIPS, Okazaki. 2001 Postdoctral Fellow, NIPS. 2002 Research fellow, OIB.
Speciality: Solid State Physics, Electron Microscopy |
 |
Postdoctoral Fellow:
UCHIDA, Hitoshi, PhD
1992 Graduated from Nihon University. 2004 Completed the doctoral course in Applied Chemistry, Nagoya Institute of Technology. 1994 AISIN CHEMICAL CO.,LTD. 2004 Postdoctoral Fellow, OIB.
Speciality: Organic Synthesis, Ceramics Chemistry |
 |
Postdoctoral Fellow:
HAYASAKA, Takahiro, PhD
1997 Graduated from Shibaura Institute of Technology. 2003 Completed the doctoral course in Functional Control Systems. 2003 Postdoctoral Fellow, JST. 2004 Postdoctoral Fellow, Mitsubishi Kagaku Institute of Life Sciences. 2004 Postdoctoral Fellow, OIB.
Speciality: Biochemistry |
 |
Postdoctoral Fellow:
SUGITANI, Shouzou, PhD
1997 Graduated from University of Tokyo. 2002 Completed the doctoral course in Arts and Sciences, University of Tokyo. 2002 Postdoctoral Fellow, OIB.
Speciality: Biophysics, Electron Microscopy |
 |
Postdoctoral Fellow:
KITAYAMA, Atsushi, PhD
1989 Graduated from The University of Tokyo, School of Engineering. 1994 Research Associate, The University of Tokyo, School of Engineering. 2000 Postdoctral Fellow, National Institute for Basic Biology, 2004 Postdoctral Fellow, OIB.
Speciality: Biotechnology |
 |
Postdoctoral Fellow:
SHIGEMATSU, Hideki,
1994 Graduated from Tokyo Institute of Technology. 1999 Completed the doctoral course in Biotechnology, Tokyo Institute of Technology. 1999 Postdoctral Fellow, NIBH, 2000 Postdoctral Fellow, Kirin Brewery Co., Ltd., 2002 Postdoctral Fellow, JST, 2003 Research Associate, Tokyo Institute of Technology, 2005 Postdoctral Fellow, OIB.
Speciality: Bioengineering, Protein Engineering |
 |
Postdoctoral Fellow:
YASUTA, Hirofumi, PhD
1992 Graduated from Faculty of Physics, Ibaraki University. 1999 Completed the doctoral course in Science and Engineering, Ibaraki University. 2000 Postdoctoral Fellow, Theory Division, Institute of Particle and Nuclear Studies, High Energy Accelerator Research Organization. 2005 Postdoctoral Fellow, OIB.
Speciality: Theoretical Physics, Quantum Field Theory |
 |
Postdoctoral Fellow:
NITTA, Koji,
2000 Graduated from University of Saitama, Faculty of Science. 2005 Completed the doctoral course in Science and Engineering, University of Saitama. 2005 Postdoctoral Fellow, OIB.
Speciality: Plant Cell Biology |
We mainly investigate molecular mechanisms of nociception and thermosensation by focusing on TRP ion channels. We also investigate signal transductions and channels involved in cell adhesion and cell movement in mammalian cells. Molecular cell biological, biochemical, developmental biological and electrophysiological techniques are utilized to achieve the above objectives. The followings are major projects in progress.
Molecular mechanisms of nociception: Capsaicin receptor TRPV1 and TRPA1 are ion channels activated by different noxious stimuli. We try to clarify the nociceptive mechanisms at peripheral nerve endings by focusing on TRP ion channels, especially TRPV1 and TRPA1. We are also doing behavioral analyses of TRPV1-deficient mice.
Molecular mechanisms of thermosensation: Temperature sensing ability is conferred by ion channels of the TRPV, TRPM and TRPA families. We try to clarify the molecular mechanisms of thermosensation by focusing on those thermosensitive TRP channels. We are also doing behavioral analyses of TRPV3- or TRPV4-deficient mice. Furthermore, we are trying to isolate a novel thermosensitive channels.
Molecular mechanisms involved in cell adhesion and cell movement: We try to understand underlying mechanisms for cell motility by focusing on signal transduction cascade involving Rho and its related proteins. We are also trying to isolate a novel mechanosensitive ion channels involved in cell adhesion and cell movement.
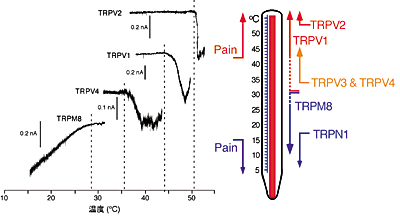 |
[Mammalian thermosensitive TRP channels: their temperature thresholds for activation (right) and activated currents (left)]
Capsaicin receptor TRPV1, TRPV2, TRPV4 or TRPM8 is activated over 43°C, over 52°C, over 36°C or under 28°C, respectively. |
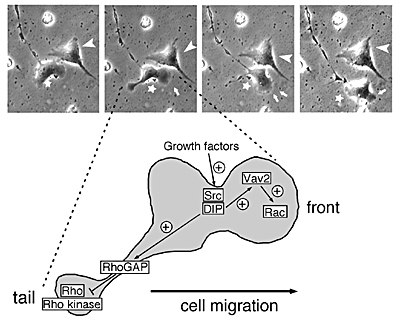 |
[Role of DIP in cell motility]
(Upper Photos) Normal fibroblast ( )moved well with membrane ruffles (arrows), although the dominant negative DIP expressing cell failed to move (arrowhead). (Lower) The model of cell migration induced by the downstream signaling of DIP. )moved well with membrane ruffles (arrows), although the dominant negative DIP expressing cell failed to move (arrowhead). (Lower) The model of cell migration induced by the downstream signaling of DIP. |
Staff
 |
Professor:
TOMINAGA, Makoto, MD, PhD
1984 Graduated from Ehime University, Faculty of Medicine. 1992 Graduated from Kyoto University Graduate School of Medicine. 1993-1999 Assistant Professor, National Institute for Physiological Sciences. 1996-1999 Research Fellow, University of California at San Francisco. 1999-2000 Assistant Professor, University of Tsukuba. 2000-2004 Professor, Mie University School of Medicine. 2004 Professor, Okazaki Institute for Integrative Bioscience.
Specialty: Cellular and Molecular Physiology |
 |
Associate Professor:
TOMINAGA, Tomoko, MD, PhD
1984 Graduated from Ehime University, Faculty of Medicine. 1990-1993 Research Fellow, Kyoto University. 1993-1995 Research Fellow, National Institute for Basic Sciences and National Institute for Physiological Sciences. 1995 Assistant Professor, National Institute for Physiological Sciences. 1996-1999 Research Fellow, University of California at San Francisco. 1999-2000 Associate Professor, Dokkyo University School of Medicine. 2003-2004 Assistant Professor, Mie University School of Medicine. 2004 Associate Professor, Okazaki Institute for Integrative Bioscience.
Specialty: Cellular and Molecular Biology |
 |
Assistant Professor:
SHIBASAKI, Koji, Ph.D.
1996 Graduated from Utsunomiya University, Faculty of Agriculture. 2001 Graduated from The Graduated University for Advanced Studies, School of Life Science, 2001-2002 Research Associate, National Institute for Physiological Sciences. 2002-2004 Research Fellow, University of Rochester School of Medicine. 2004 Assistant Professor, Okazaki Institute for Integrative Bioscience.
Specialty: Cellular and Molecular Neurobiology |
 |
Research Fellow:
INADA, Hitoshi, PhD
1998 Graduated from Kyushu University Graduate School of Science. 1998-1999 Postdoctoral Fellow, Kyushu University. 1999-2005 Postdoctoral Fellow, Nagoya University. 2005 Research Fellow, Okazaki Institute for Integrative Bioscience.
Specialty: Molecular Neurobiology, Behavioral Genetics |
 |
Research Fellow:
SOKABE, Takaaki, PhD
1998 Graduated from Himeji Institute of Technology, Faculty of Science. 2004 Graduated from University of Tokyo Graduate School of Medicine. 2004-2005 Research Fellow, University of Tokyo Graduate School of Medicine. 2005 Research Fellow, Okazaki Institute for Integrative Bioscience.
Specialty: Cellular and Molecular Biology |
 |
JSPS Postdoctoral Fellow:
MANDADI, Sravan, PhD
1995 Graduated from Berhampur University, Faculty Pharmacy (India). 2004 Graduated from University of Sydney, Faculty of Pharmacy (Australia). 2005 JSPS Postdoctoral Fellow.
Specialty: Neurophysiology |
|





 )moved well with membrane ruffles (arrows), although the dominant negative DIP expressing cell failed to move (arrowhead). (Lower) The model of cell migration induced by the downstream signaling of DIP.
)moved well with membrane ruffles (arrows), although the dominant negative DIP expressing cell failed to move (arrowhead). (Lower) The model of cell migration induced by the downstream signaling of DIP.



























