|
|
|
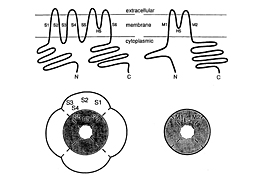
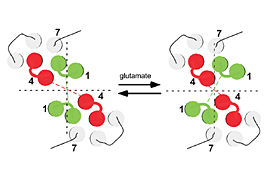
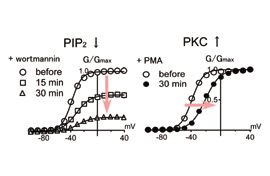
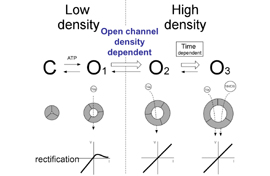
DEPARTMENT OF MOLECULAR PHYSIOLOGYDivision of Biophysics and NeurobiologyIon channels, receptors and G proteins play critical roles for the excitability and its regulation of neurons. We focus on these molecules which enable brain function. From the biophysical point of view, we study structure-function relationships, regulation mechanisms and dynamic structural rearrangements of ion channels and receptors. We also plan to study the functional significance of specific features of ion channels and receptors in the brain function by making knock-in mice and by studying their abnormalities in the synaptic transmission and whole animal behavior. Specific themes of research projects currently running are as follows
|
|||||||||||||
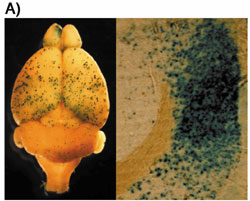
Division of Neurobiology and BioinformaticsDuring the course of formation of the mammalian central nervous system, neuroepithelial cells differentiate into various kinds of cells to make a fine three-dimensional network. Our goal is to understand genetic control over these processes. As a first step, we have cloned several genes that are specifically expressed in a certain type of brain cells and are investigating their role on cell fate determination. Neural cells are known to leave the ventricular zone after their commitment, and migrate towards destinations. While radial neuronal migration has been studied extensively in the developing cerebral and cerebellar cortices, mechanisms underlining tangential migration of neuronal and glial progenitors remains unclear. We are employing in ovo or in utero electroporation method to introduce exogenous genes in developing central nervous system, and studying mode and mechanisms of neural cell migration. We are making use of hereditary mutant mice that exhibit abnormal development of the nervous system. We also use in situ hybridization and immunohistochemical technique to study cell lineages during development of the nervous system. Neural stem cells, which are ultimate lineage precursors to all neurons and glia in the mammalian brain, are present not only in embryonic but also in adult brains, and contribute to adult neurogenesis. We are investigating molecular mechanisms underlying the generation, proliferation, maintenance, differentiation, and senescence of the neural stem cells, which will clarify their in vivo kinetics and function. An automated system to analyze N-linked sugar chains was developed to study their biological roles during development and tumorigenesis. New retroviral vectors are also constructed for efficient gene delivery, which will be used for cancer gene therapy. |
|
 |
 |
|
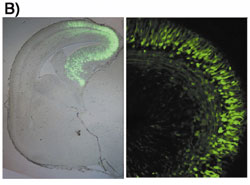
In Utero Gene Transfer Systems to the Embryonic Mouse Brains B) In utero electroporation was carried out for plasmid DNA transfer. Green fluorescent protein (GFP) expression vector was injected into lateral ventricle and electroporated in utero. The cells in the restricted region were observed to express GFP | |
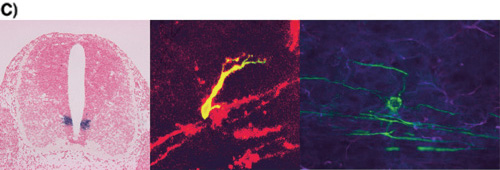
C) Oligodendrocyte Development(Left) pMN domain which is the site of oligodendrogenesis Expression of Olig2 gene in embryonic day 12 spinal cord. Olig2 (purple) is expressed ventral ventricular zone called pMN domain.(Middle) Migrating oligodendrocyte progenitor Oligodendrocyte progenitor is double-stained by anti-GFP antibody (green) and O4 antibody (red). O4 is an oligodendrocyte lineage specific marker(Right) Myelinating oligodendrocyte Mature oligodendrocyte (green) is observed with extending processes toward several axons. |
Division of Intracellular MetabolismCell signaling that generates proper cell responses to various stimuli is the essence of life. To understand its mechanism is one of the goals of life sciences. This division is aiming to elucidate the spatio-temporal regulation mechanisms underlying cell signaling, focusing on the dynamics of ion channels, cytoskeletons, and adhesion molecules by use of electrophysiological and advanced imaging techniques. The subjects of research are, (1) Cell signaling in response to mechanical stimuli: (2) Intracellular Ca2+ signaling: (3) Proton signaling: |
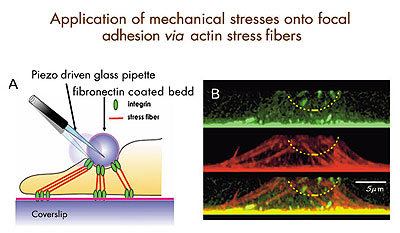
Fig.1: Diagram for mechanical stimulation of focal adhesions through stress fibers. Left: Fibronectin-coated glass beads connected to the basal focal adhesions via stress fibers. By displacing the bead, we can apply localized mechanical stimuli onto focal adhesions, while recording the surface dynamics of intracellular calcium and integrin by near field microscopy. Right: Projected side views of focal adhesions (top, green spots), stress fibers (middle, red strands), and their superimposition (bottom) in an endothelial cell . |
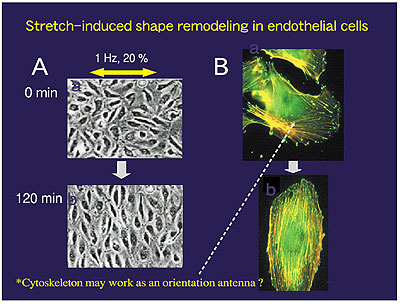
Fig.2: Stretch-induced shape remodeling. Left: When subjected to uniaxial cyclic stretch, endothelial cells cultured on an elastic silicone membrane change their shape from cobble stone-like to spindle-like by aligning their long axis perpendicular to the stretch axis. Right: Dynamic rearrangement of focal adhesions (green spots) and stress fibers (orange strands) before (top) and after (bottom) remodeling. |
|
|
|
| Copyright(C) 2005 NIPS (www-admin@nips.ac.jp) | |