SUPPORTIVE CENTER FOR BRAIN RESEARCH
This center has been activated as the "Center for Brain Experiment" until the end of March 2008. Then, to expand its role in supporting brain research at NIPS, the center was reorganized as the "Supportive Center for Brain Research" in April 2008. This center is comprised of six sections, Section of Brain Structure Information, Brain Function Information, Multiphoton Neuroimaging, Electron Microscopy, Instrument Design, and Ine Marine Laboratory. The latter three sections were combined with this center in this April.
Brain research is one of the hottest scientific topics worldwide, of course including Japan, and recent progress in brain research has been very surprising and attractive. Brain research is one of the main themes at NIPS and recently NIPS has been reorganized as one of the most advanced centers for brain research in Japan. The main objective of this center is to support brain research performed at NIPS. Following the reorganization of this center, we have become better able to support brain research in various fields at NIPS.
Director
 |
Professor:
KAKIGI, Ryusuke, MD, PhD
1978 Graduated from Kyusyu University, Faculty of Medicine. 1981 Clinical Associate, Department of Internal Medicine, Saga Medical School. 1983-1985 Research Fellow, The National Hospital for Nervous Diseases, University of London. 1992 Assistant Professor, Department of Internal Medicine Saga Medical School. 1993 Professor, NIPS.
Speciality: Neurophysiology |
The ultimate object of this laboratory is to clarify the relations between structures and functions in the brain. To study them, a high voltage electron microscope (H-1250M) which is specially designed for biological and medical research is available since 1982. The daily accelerating voltage of the microscope is 1,000kV. The pressure near the specimen position is less than 7X10-6Pa and the magnification ranges 1k to 1,000k times. Transmission images of thick specimens till about 5mm can be taken.
Since this is the only high voltage electron microscope in the biological field in Japan, the collaborative programs are carried out by about 15 research groups from Universities etc. on three projects: 1) Three-dimensional analysis of fine structures of biological specimens 2) High resolution observation of biological specimens 3) Observation of biological specimens in their natural conditions.
Facilities for tissue culture and light microscopy are also provided. Cryostats, microtomes and fluorescence microscopes with a high-resolution colour cooled CCD camera system are prepared for immunohistochemistry. Inverted microscopes with a time lapse video system are prepared to observe cultured cells.
 |
High voltage electron microscope (H-1250M: 1,000kV)
Specially designed for the exclucive use of medical and biological specimens |
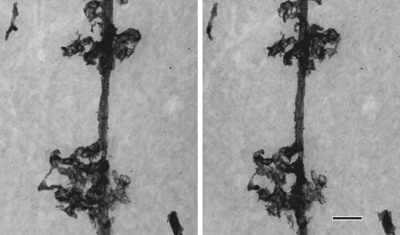 |
Cell processes of a Bergmann glia in the rat cerebellum revealed by Golgi staining
Stereo images taken at ±8º tilt at 1,000kV. Specimen thickness: 3mm. Scale bar: 2mm. |
Staff
 |
Associate Professor:
ARII, Tatsuo, PhD
1967 Graduated from Tohoku University, Faculty of Science. 1972 Completed the doctoral course in Engineering, Nagoya University. 1972 Research Associate, Nagoya University. 1973 Research Associate, Regensburg University. 1976 Research Associate, Nagoya University. 1979 Associate Professor, NIPS.
Speciality: Electron Microscopy |
 |
Assistant Professor:
FURUYA, Sonoko, PhD
1970 Graduated from University of Tokyo, Faculty of Pharmacy. 1975 Completed the doctoral course in Pharmacy, University of Tokyo. 1975 Research Associate, Nihon Medical College. 1978 Research Associate, NIPS.
Speciality: Tissue Culture and Histology |
In order to investigate the brain mechanism underlying our mental ability such as cognition, voluntary movement, thinking, or will, we have to experiment on the human brain. Some non-invasive techniques for measuring brain are certainly useful for the purpose. However, they are still insufficient in the quality of information. To overcome the limitations, researches on the brain are carried out here in both the human and monkey subjects using various techniques including direct recordings of cortical field potential, magnetoencephalography, and positron emission tomography.
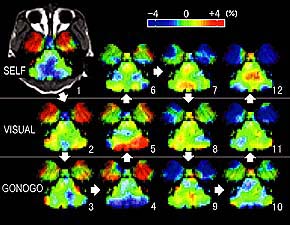
Figure 1. Brain activity during three kinds of movement tasks (a specimen record in one day).
The monkey was engaged in hand movement tasks to get a reward in three different sets of circumstances; i.e. the self-initiated movement task (SELF), the visually-initiated movement task (VISUAL), and the color-discriminating go/no-go task with asymmetrical reinforcement (GONOGO). Arrows and numbers indicate the order in which they were taken. Although the activity fluctuates, a statistical analysis across repetitive measurements can extract the significant activity changes (Figures 2, 3, and 4) |
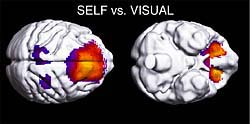
Figure 2. Regions of activated in SELF task.
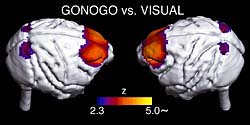
Figure 3. Regions activated in GONOGO task.
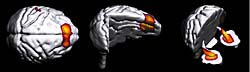
Figure 4. Regions of significant decrease in activity during task repetition in a day.
The decline in the activity of the limbic and prefrontal regions may be a reflection of a decline in the willingness.
|
Staff
 |
Associate Professor:
TSUJIMOTO, Toru, MD, PhD
1986 Graduated from Kyoto University, Faculty of Medicine. 1990 Completed the doctoral course in Medicine, Kyoto University. 1993 Research Associate, NIPS. 1994 Research Associate, Kyoto University. 1999 Associate Professor, NIPS.
Speciality: Neurophysiology
|
We have developed one of the most advanced "two-photon microscopy" (Figs. 1-3). We support international or domestic collaborative researches using this novel microscopy. The microscopy has given us important insights into secretory functions in neural and secretory gland cells. We have especially proved the existence of "sequential compound exocytosis" (Fig. 2).
We explore two-photon microscopy by incorporation of photo-activated probes, molecular biologiy, patch-clamp techniques, and non-linear optical techniques. Our newly constructed "in vivo" two-photon microscopy enables us to obtain a complete picture of a living mouse neuron (Fig. 1). In addition, we have developed a new super-resolution method to accurately determine vesicle diameter below the diffraction limit of light.
The goal is to reveal "missing-links" underlying between molecular functions and physiological functions in a living body. Spatiotemporal dynamics of biomolecular interactions in situ should be demonstrated for the elucidation of physiological functions, including neural or glial activities, and secretion. Such investigation is also critical for the elucidation of biophylaxis or development. We have thus advanced new optical methods of "visualization by photon" and "manipulation by photon".
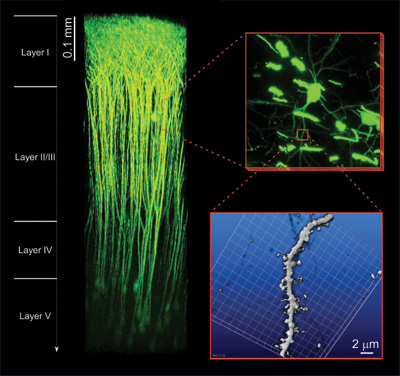 |
| Figure 1. "In vivo" two-photon microscopy. The superior tissue penetration of our newly constructed "in vivo" two-photon microscopy can visualize neural activities in the living brain. EYFP fluorescence can be detected from layers deeper than 0.9 mm beneath the brain surface in an anaesthetized mouse. 3D-reconstruction of individual neural cells spreading in the layers I-V is available without any degradation of sub-micron spatial resolution. (Collaborative research with Prof. J. Nabekura. |
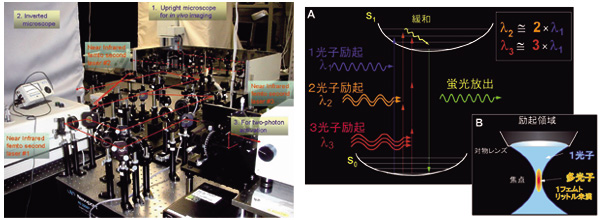 |
| Figure 2. Multi-photon excitation process. By using near infrared femto-second laser, multi-photon excitation of molecules is elicited through simultaneous absorption of photons (A) at the focal point of an objective lens (B). Two-photon excitation imaging (two-photon microscopy) has deeper tissue penetration, little out-of-focal light absorption and least phototoxic effects. It is thus quite suitable for investigating molecular and cellular events within thick intact tissues. Moreover, it allows simultaneous multi-color imaging and fluorescence correlation measurement. The fusion pore opening and its dynamics can be resolved with nanometer order (Nature Cell. Biol., 3: 253, 2001, Science, 297:1349, 2002). |
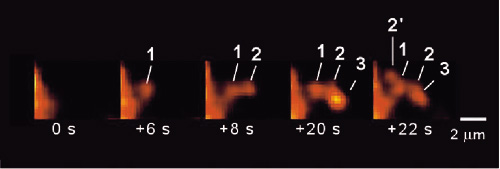 |
| Figure 3. The discovery of "sequential compound exocytosis". Two-photon microscopy has demonstrated sequential progression of exocytosis deep into the cytosol in exocrine glands. Such sequential compound exocytosis is now considered to be utilized commonly for physiological secretions in a wide variety of cells and organs (Nature Cell. Biol., 3:253, 2001, EMBO J, 25:673, 2006). |
Staff
 |
Professor:
NABEKURA, Junichi, MD, PhD
1980 Graduated from Kyushu University, School of Medicine. 1986 Completed the doctoral course in Medical Sciences, Kyushu University. 1986 Research Fellow, Washington University. 1991 Assistant Professor, Department of Neurophysiology, School of Medicine, Tohoku University. 1993 Associate Professor, Department of Physiology, School of Medicine, Akita University. 1995 Associate Professor, Kyushu University, Graduate School of Medical Sciences. 2003 Professor, NIPS.
Speciality: Neuroscience |
 |
Associate Professor:
NEMOTO, Tomomi, PhD
1991 Graduated from, Department of Physics, Faculty of Science, the University of Tokyo. 1996 Completed the doctoral course in Applied Physics in Tokyo Institute of Technology. 1996-1997 Frontier Researcher and Special Postdoctoral Researcher, RIKEN. 1997-1999 Research fellow, the University of Tokyo. 1999-2005, Assistant professor, NIPS. 2001-2004, Researcher, PRESTO, JST.
Specialty: Cell physiology, Biophysics |
|
The "Ine Marine Laboratory (IML)" was founded in February 1986 as a facility for physiological experiments on mollusks such as squid and Aplysia. The IML is located on the coast in Ine Bay (a part of Wakasa bay) in the northern part of the Tango Peninsula (Kame-shima, Ine, Kyoto). The Laboratory is situated in a secluded bay outside the ancient traditional fishing village of Ine. The beautiful coastline and countryside surrounding the laboratory make it a delightful location for research and study. The IML has two well-equipped laboratory rooms, a small machine shop, a small seawater tank room, three bedrooms, a kitchen, and a bathroom with laundry facilities, providing enough space for a several scientists to live and work together. Ine bay has a diverse fauna of marine organisms including, for example, fish, mollusks, starfish, sea urchins, and a variety of plankton species.
|

The Ine Marine Laboratory
(Kame-shima, Ine, Kyoto) |
 |
Assistant Professor (NIPS):
KUKITA, Fumio, PhD
1971 Graduated from the University of Tokyo, Faculty of Science. 1976 Completed the doctoral course in physics at the University of Tokyo, 1977 Research Associate, NIPS.
Speciality: Biophysics and Molecular Physiology |
|
















