|
|
|
DEPARTMENT OF INFORMATION PHYSIOLOGYThe main purpose of this division is to study the neural mechanisms of visual perception. The human visual system is a complicated parallel and distributed system where several neural structures play different roles, but are still able to generate a unified and integrated precept of the outer world. This system also has sophisticated mechanisms that enable reconstruction of three-dimensional structures from two-dimensional retinal images. To understand the neural substrates of these abilities in our visual system, we are recording neuronal activities from the primary visual cortex and extrastriate visual areas. We are analyzing the stimulus selectivity of neurons to determine the representation of various kinds of visual features, suchas color, motion, shape and depth. We are also studying the dynamics of visual information processing in the cortex by analyzing the temporal pattern of neural activities. In addition, to explore the ways in which various visual features contribute to visual perception, psychophysical experiments are conducted in this laboratory. 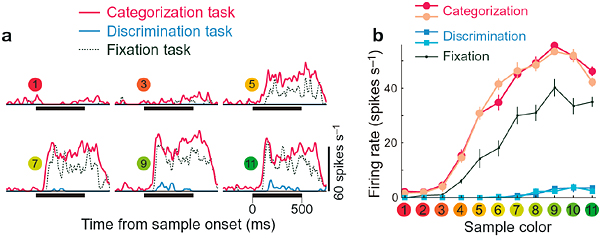 Two different functions can be distinguished in our color vision; one is categorization, the other is fine discrimination. These functions are utilized depending on the task demand, and we found that activities of many neurons in the inferior temporal cortex of the monkey to the same color stimulus changes depending on the task demands.Many neurons showed stronger responses when the monkey makes categorical color judgement than when it makes fine discrimination. This figure shows the responses of one such example of neuron. Responses during categorization, discrimination and fixation tasks are shown as histograms in a and as line graphs in b.
Staff
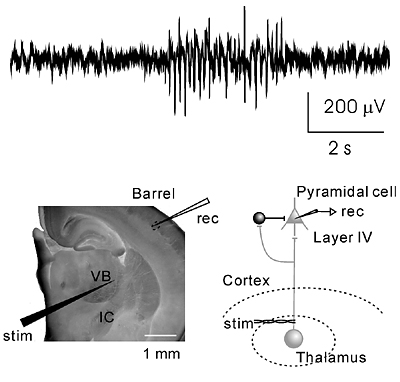
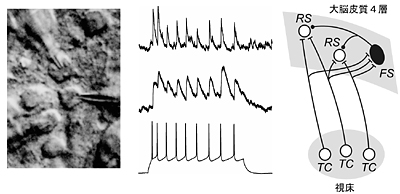
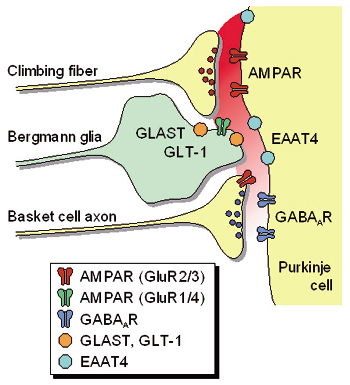
Our main interest lies in elucidation of the mechanism of transduction and integration of neural information in the nervous system. More specifically, we are trying to understand the basic properties of neural information processing between neurons or among a group of neurons constituting a local network. We are also interested in the pathophysiological mechanism how a single gene mutation leads to a symptom (such as ataxia and epilepsy), particularly in Ca2+ channel mutant mice. Additionally, we have recently started to make a computational approach, incorporating computer-based neurons into brain slice measurements (dynamic clamp), together with computational simulation of network functions. The following are currently ongoing major projects. (1) Studies of neurological disorders caused by calcium channel mutations. Mutations of the voltage-gated calcium channels are associated with neurological disorders of human and mice, which include cerebellar ataxia and some forms of seizure disorders. We study the relation how a single mutation causes neurological manifestations, mainly using brain slice preparations. (2) Integration of sensory inputs in the thalamus. Recent studies revealed the mechanism of processing the sensory information at the peripheral nerves and the spinal cord, but little is known about the operational mechanisms in the thalamus. To understand the information processing by groups of neurons, we identified the wiring diagrams among the neurons in the thalamus and layer 4 cortical neurons (Fig. 2). We also studied the feedback projection from the cerebral cortex to the thalamus, and showed that there are significant differences in synaptic properties between inputs from the peripheral and from the cerebral cortex. (3) Transmitter diffusion-mediated crosstalk between heterologous neurons. In principle, neuronal information is unidirectionally transported at the synapses, however, recent studies suggested that synaptic transmission can be mediated by retrogradeand/or heterosynaptic pathways. We reported that the excitatory transmitter glutamate diffused from climbing fiber (CF) terminals [projection to cerebellar Purkinje cells (PCs) from the inferior olive in the brain stem] presynaptically suppressed the inhibitory information flow from basket cells to PCs. The heterosynaptic inhibition is mediated through the activation of AMPA receptors in the presynaptic terminals of basket cells. The heterosynaptic inhibition therefore provides a likely mechanism boosting the CF input-derived excitation to the PCs (Fig. 3).
Staff
Diverse types of membrane proteins act in concert to form excitabilities in neurons and muscle cells. We are focusing on mechanisms and physiological roles of novel voltage-sensing proteins that we discovered in the last few years. We also study mechanisms of neuronal differentiation that underlies expression of neural functions such as fish locomotion. 1.Physiological role of membrane potential and novel voltage-sensing proteins Voltage sensors have long been thought as traits unique to voltage-gated ion channels that underlie membrane excitabilities. We have recently identified a novel protein that contains voltage sensor similar to ion channel and phosphatase. This protein shows voltage-dependent tuning of phosphatase activity based on the operation of voltage sensor. This indicates that voltage sensing is not confined to ion channels but could be more widespread than previously realized. We are currently asking how voltage sensor operation is coupled to phosphatase activity, and what kind of biological context this protein is involved. We have recently identified another voltage sensor domain protein, VSOP, that lacks cytoplasmic region. Surprisingly, this protein exhibits voltage-gated proton channel activities when expressed heterologously. This channel is abundantly expressed in blood cells such as macrophage and neutrophil, and could play role in regulation of production of reactive oxygen species and intracellular pH. The roles of membrane potential and pH in respiratory burst are being investigated. We also want to understand how both sensing membrane potential and regulation of proton permeation is achieved by this small molecule. 2.Mechanisms of integration of ion channels and other membrane proteins We aim to reveal how ion channel expression and functions are integrated into physiological functions such as phagocytosis, neural functions and development. 3,Neuronal basis of locomotion and its development Recent molecular genetic studies suggest that the expression of transcription factors in the developing spinal cord helps determine the morphological and physiological properties of neurons. Using the zebrafish preparation, we have been examining the electrophysiological and morphological properties of neurons specified by individual or sets of transcription factors. 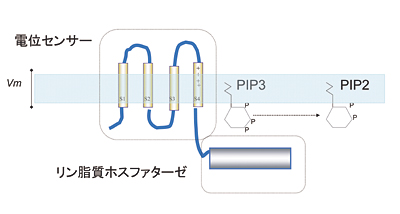 Voltage-sensing phosphatase Ci-VSP is a protein that contains channel-like voltage sensor and phosphatase. Phosphatase activity is tuned by the operation of its intrinsic voltage sensor in response to change of membrane potential. This provides a signaling pathway coupled with membrane potential change without requiring ionic flow. 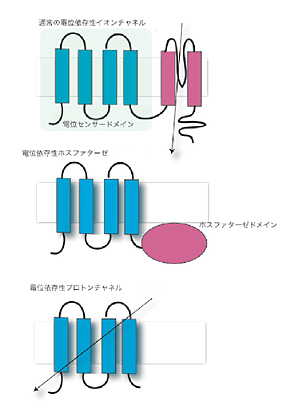 A family of "voltage-sensor domain proteins" Ci-VSP and VSOP belong to the voltage-sensor domain proteins. These lack pore domain and voltage-sensor domain operates by itself either by a voltage sensor or a voltage-gated proton channel.. Biological roles of voltage sensor is more widespread than previously appreciated. 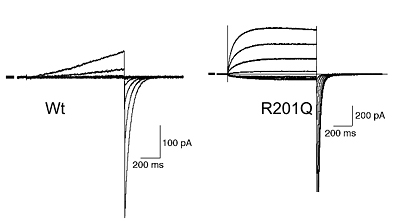 Patch clamp recording of voltage-gated proton currents from tsA201 cells transfected with mouse VSOP cDNA Voltage-gated proton currents recorded in the whole-cell patch recording mode. Wt shows outward-rectifying currents and tail inward currents. R201Q mutant shows inward currents during depolarization due to the altered voltage-dependency. 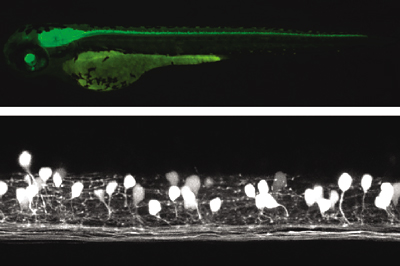 Studies with zebrafish as a model system to understand molecular mechanisms underlying development of neuronal wiring and neurophysiology of locomotion. In the transgenic zebrafish, a class of inteneurons are easily identified by fluorescence of GFP in live animals. The upper panel is an image using a regular fluorescent micoscope. The bottom panel is an image by a confocal microscopy. Staff
| ||||||||||