DEPARTMENT OF DEVELOPMENTAL PHYSIOLOGY
Outline
Department of Developmental Physiology was founded in 2003 with 3 full-staff divisions and 1 adjunct division, for the purpose of clarifying the physiological mechanism of mental and physical development of human beings. One of the divisions was transferred from the previous Higher Brain Function Project, Department of Integrative Physiology, headed by Prof. Tadashi Isa. This division (Division of Behavioral Development) studies the development and post-injury recovery of the neural systems controlling the eye and hand movements. Prof. Junichi Nabekura was elected as a chair of the Division of Homeostatic Development and has initiated the activity of the division since 2003. This division studies the development of synapses in the central nervous system and remodeling of neural circuits after the brain injury by using electrophysiological techniques and imaging with multi-photon microscopes. Prof. Yasuhiko Minokoshi was elected as the chair of Division of Reproductive/Endocrine Development, and started researches on neural control of metabolism, especially focused on the function of the hypothalamus, to obtain better understanding of the molecular mechanisms of pathophysiology of obesity and diabetis mellitus. Division of Adaptation Development, an adjunct division, had been chaired by Prof. Hideto Kaba of Kochi University, whose is a specialist on the adaptive mechanism of the reproductive behaviors guided by olfacation, since 2003, but his term was expired at the end of FY2008 and the successor is now in the process of election.
We are investigating the neural systems controlling saccadic eye movements and grasping hand movements. We analyze the structure and function of local circuits and large-scaled networks involved in these motor systems. We are also interested in plastic compensatory mechanism following lesion of related structures as detailed below;
1. Saccadic eye movements
(1)Electrophysiological analysis of local circuits of the superior colliculus (SC), a pivotal midbrain center for saccade control, by using in vitro slice preparation.
(2)Analysis of local circuit of SC and large scaled network involving the cerebral cortex for saccade generation in anesthetized animals (rodents and non-human primates).
(3)Molecular mechanism of saccade generator circuits by using genetically manipulated mice.
(4)Analysis of dynamic properties of saccade-related circuits by applying electrophysiological and pharmacological techniques in awake behaving non-human primates.
(5)To clarify the neural mechanism of saccade control and visual awareness in "blindsight" patients, we are analyzing the saccadic behaviors and neuronal activities in macaque monkeys with unilateral lesion of the primary visual cortex (V1), as an animal model of "blindsight".
2. Dexterous hand movements
Recently we clarified the existence of oligosynaptic (indirect) pathway from the primary motor cortex to hand motoneurons mediated by interneurons in the cervical spinal cord. Moreover, we observed the behavior of the monkeys in which direct cortico-motoneuronal connections are transected while the indirect pathway remained intact and found that the monkeys can perform precision grip after 1-3 months of recovery period. To explore the basic mechanism involved in the compensatory mechanisms, we use multidisciplinary approaches including electrophysiology, non-invasive brain imaging with positron emission tomography (PET) and analysis of gene expression by DNA microarray and in-situ hybridization.
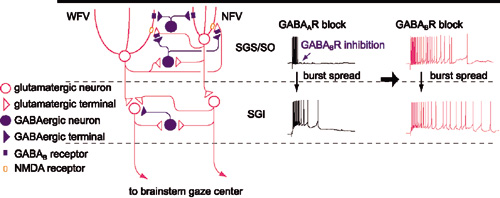
Figure 1. Schematic of local circuit underlying GABABR-mediated regulation of bursts in the SC. Postsynaptic GABABRs expressed both in NFV and WFV cells and presynaptic GABABRs located on gluamatergic synaptic terminals in the SGS are activated by synaptically released GABA during bursts of SGS GABAergic neurons. Hyperpolarization in NFV cells, shunting inhibition in WFV cells, and reduction of glutamate release may contribute to the limitation of burst duration. When GABABRs are blocked, burst duration in the SGS may be prolonged in an NMDAR-dependent manner, and then, the prolonged burst may spread to the SGI. Thus the burst duration of the SGI may also be prolonged.
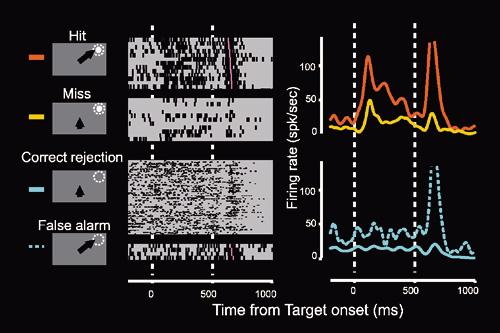
Figure 2. Neuronal activities in the SC ipsilateral to V1 lesion correlated to the monkey's performance. When the monkey succeed to detect them ('Hit' trial), this neuron exhibited clear visual responses to the visual stimuli presented in the visual field affected by the lesion, while when the monkey failed to detect them ('Miss' trial), these responses attenuated although the same visual stimuli were presented.
Figure 3. Increased brain activation during the recovery of precision grip after injury of the corticospinal tract at the cervical segments detected by PET scanning in macaque monkeys. Increased brain activation during the early recovery period (1 month after injury; left) was mainly observed in bilateral primary motor cortex, and during the late recovery period (3 months; right) it was observed in the contralateral primary motor cortex and bilateral premotor cortex.
Staff
 |
Professor:
ISA, Tadashi, MD, PhD
1985 Graduated from University of Tokyo, Faculty of Medicine. 1989 Completed the doctoral course in Science in University of Tokyo. 1989 Research Associate in University of Tokyo. 1993 Lecturer in Gunma University, School of Medicine. 1996 Professor, NIPS.
Speciality: Neurophysiology |
 |
Assistant Professor:
SEKI, Kazuhiko, PhD
1988 Graduated from Niigata University, Faculty of Education. 1998 Completed the doctoral course in Tsukuba University, Faculty of Medicine. 1998 Lecturer in International Budo University, Postdoctoral fellow in University of Washington (Seattle). 2001 Research Associate, NIPS.
Speciality: Neuroscience |
 |
Assistant Professor:
YOSHIDA, Masatoshi, PhD
1992 Graduated from University of Tokyo, Department of Pharmaceutical Sciences.1996 Predoctoral Fellow at NIPS and University of Tokyo. 2003 Obtained PhD from University of Tokyo. 2003 Postdoctoral Fellow at University of Tokyo. 2003 Research Associate, NIPS.
Speciality: Cognitive Neuroscience and Neurophysiology |
 |
Assistant Professor:
KANEDA, Katsuyuki, PhD
1994 Graduated from Kyoto University, Department of Pharmaceutical Sciences. 1999 Completed the doctoral course in Graduate School of Pharmaceutical Sciences, Kyoto University. 1999 Postdoctoral Fellow in Tokyo Metropolitan Institute for Neuroscience. 2003 Postdoctoral Fellow in University of Tennessee. 2005 Research Associate, NIPS.
Speciality: Neurophysiology and Neuropharmacology |
 |
Postdoctoral Fellow:
WATANABE, Hidenori, PhD.
1997 Graduated from Tokyo Univ. of Science, Dept. of Physics. 2002 Completed the doctoral course in The Univ. of Tokyo, S. of Engineering. Research Fellow, Tamagawa Univ.. 2003 JSPS Research Fellow. 2005 Research Fellow, Tamagawa Univ.
Speciality: Neuroscience, Information systems engineering. |
 |
Postdoctoral Fellow:
SAKATANI, Tomoya, PhD
1997 Graduated from the University of Tokyo, Faculty of Science. 2004 Obtained PhD from the Graduate University for Advanced Studies (SOKENDAI). 2004 Postdoctral Fellow at NIPS. 2005 Visiting Fellow at the University of Oxford. 2006 Postdoctral Fellow at NIPS.
Speciality: Neuroscience |
 |
Postdoctoral Fellow:
KATO, Rikako, PhD
1997 Graduated from Ibaraki University, Faculty of Science. 2003 Completed the doctoral course in Tsukuba University, Faculty of Medicine. 2003 Postdoctral Fellow, NIPS. 2003 Postdoctral Fellow, College de France. 2005 Postdoctral Fellow, NIPS.
Speciality: Neuroscience |
 |
Postdoctoral Fellow:
UMEDA, Tatsuya, PhD
1998 Graduated from University of Tokyo, Faculty of Science. 2004 Completed the doctoral course in Tokyo Medical and Dental University, Faculty of Medicine. 2005 Postdoctral Fellow at Tokyo Medical and Dental University, 2007 Postdoctral Fellow, NIPS.
Speciality: Neurophysiology |
 |
Postdoctoral Fellow:
IKEDA, Takuro MD, PhD
1998 Graduated from Tokyo University, Faculty of Science. 2004 Completed the doctoral course in University of Tokyo, Faculty of Medicine. 2005 Postdoctral Fellow, NIPS.
Speciality: Neurophysiology and Cognitive Neuroscience |
 |
Postdoctoral Fellow:
KIM, Gee Hee, PhD
2001 Graduated from Waseda University, Faculty of Human Sciences. 2006 Predoctoral Fellow at Research Institute, National Rehabilitation Center for persons with Disabilities. 2007 Obtained PhD from Waseda University. 2008 Postdoctoral Fellow at NIPS.
Speciality: Neurophysiology |
 |
Postdoctoral Fellow:
TAKEI, Tomohiko, PhD
2004 Graduated from Kyoto University, Faculty of Integrated Human Studies. 2008 Completed the doctoral course in Graduate School of Human and Environmental Studies, Kyoto University. 2008 Postdoctoral Fellow at NIPS.
Speciality: Neurophysiology |
 |
Postdoctoral Fellow:
OYA, Tomomichi, PhD
2004 Graduated from Kyoto University, Faculty of Integrated Human Studies. 2009 Completed the doctorate in Neuroscience at The University of Queensland, School of Human Movement Studies in Australia. 2009 Postdoctoral Fellow at NIPS.
Speciality: Neurophysiology, Motor control |
In the last stage of neural development, a massive re-arrangement of neuronal circuits takes place. This is associated with an alteration of neuronal circuits which is already functioned, resulting in the changes in various brain functions e.g. behavior, sensory function and biological rhythm. Our research aim is to understand the developmental re-arrangement of brains function at neuronal circuits and synaptic levels by using various electrophysiological methods and molecular approach. In addition, their modulation by the neural activity driven by intrinsic factors and environments is also studied.
Another question is whether the neural rearrangement could re-appear during the recovery after various brain damages, e.g. brain ischemia and injury.
(1) One of our recent targets is to elucidate the developmental plasticity of GABAergic system. In immature animals, GABA induces neuronal depolarization and often acts as a excitatory substance. We study on the cellular and molecular mechanisms of the switch of GABA action from depolarization to hyperpolarization during development. Intracellular CI- concentration decreases during development, resulted from developmental switch of intracellular CI- regulators, e.g. K+ CI- cotransporter2 (KCC2) and Na+,K+,CI-cotransporter 1 (NKCC1). In addition, immediately after neuronal damages, KCC2 rapidly down-regulated in their expression and function, resulting in GABA-induced excitation in injured neurons.
(2) We recently reported a new form of synapse development. Transmitters to the lateral olive neurons, auditory relay neurons, switch from GABA in the immature to glycine in the mature. The transmitter switch proceeds with single synaptic terminals. This could be one of unique synaptic plasticity in developing neural circuits. We now focus on elucidating underlying mechanisms, e.g. related trophic factors.
(3) We also investigate the rapid action of BDNF on GABAergic system of developing visual cortex and hippocampus of rat/ mouse. BDNF rapidly upregulates the surface GABA-A receptor and induces long term enhancement of GABA-induced currents in amplitude. BDNF action switches to rapid down-regulates surface GABA-A receptor in the mature. Underlying mechanisms, including neural activity and GABA-A receptor associated protein, is now under investigation.
(4) In addition, we are interested in elucidating the underlying mechanisms for re-acquisition of cellular and functional immature characteristics in the process of recovery after the cell damage, e.g. GABA excitation resulting from rapid down-regulation of KCC2 expression after neuronal injury, and dominant expression of immature NMDA receptor subunits associated with the alteration of its function.
(5) We attempted to visualize the neuronal circuits and its fine structure by using 2 photon microscope in an in vivo animal. We currently succeed in observing fine structure of pyramidal neuron through entire layers of mouse cortex. By employing this technique, we will attempt to elucidate the mobility of neuronal spines and various glia during development and after neuronal injury. Especially, the surveillance of microglia on synapse structures and its alteration in the ischemic brain are visualized in an in vivo condition. In addition, neuronal activities are recorded in the chronic pain models using various Ca2+ fluorescent probe.
Staff
 |
Professor:
NABEKURA, Junichi, MD, PhD
1980 Graduated from Kyushu University, School of Medicine. 1986 Completed the doctoral course in Medical Sciences, Kyushu University. 1986 Research Fellow, Washington University. 1991 Assistant Professor, Department of Neurophysiology, School of Medicine, Tohoku University. 1993 Associate Professor, Department of Physiology, School of Medicine, Akita University. 1995 Associate Professor, Kyushu University, Graduate School of Medical Sciences. 2003 Professor, NIPS.
Speciality: Neuroscience |
 |
Associate Professor:
ISHIBASHI, Hitoshi, PhD
1988 Graduated from Kyushu University, Faculty of Pharmaceutical Sciences. 1990 Completed the master course in Pharmaceutical Sciences, Kyushu University. 1996 Completed the doctoral course in Medical Sciences, Kyushu University. 1998 Assistant Professor, Faculty of Pharmaceutical Sciences, Kumamoto University. 2000 Assistant Professor, Graduate School of Medical Sciences, Kyushu University. 2007 Associate Professor, NIPS.
Speciality: Neuroscience |
 |
Research Associate:
WATANABE, Miho, PhD
1996 Graduated from Waseda University, Faculty of Human Sciences. 1998 Completed the master course in Human Sciences, Waseda University. 2004 Completed the doctoral course in Medicine, Nippon Medical School. 2004 Research Fellow, NIPS. 2006 Research associate, NIPS.
Speciality: Neuroscience. |
 |
Postdoctoral Fellow:
Eto, Kei, PhD
2004 Graduated from University of Shizuoka, Faculty of Pharmaceutical Sciences. 2006 Completed the master course in Pharmaceutical Sciences, Kyushu University. 2009 Completed the doctoral course in Pharmaceutical Sciences, Kyushu University. 2007 JST Research Fellow.
Speciality: Neuroscience |
 |
Visiting Researcher:
KIM, Sun Kwang, OMD, PhD
2002 Graduated from Kyung Hee University, College of Oriental Medicine. 2008 Completed the doctoral course in Oriental Medicine, Kyung Hee University. 2008 Research fellow, Acupuncture & Meridian Science Research Center, Kyung Hee University. 2008 JSPS fellow.
Speciality: Neuroscience |
The animal body has an integrated-regulatory system for "homeostasis" that maintains a normal, constant-internal state by responding to changes in both the external and internal environments. Within the central nervous system, the hypothalamus is a critical center that regulates the homeostatic activities by integrating autonomic nervous system, endocrine system and immune function. In this division, we are extensively investigating the role of hypothalamus in body energy balance in mammals. These studies are now important for better understanding the molecular mechanisms behind pathophysiology of obesity and diabetes mellitus. The main subjects of our current research are as follows;
(1) Molecular mechanism of the hypothalamic regulation of food intake.
(2) Regulatory role of the hypothalamic-sympathetic nervous system in glucose and lipid metabolism.
(3) Signaling pathway for metabolic action of leptin and adipokinens in peripheral tissues.
(4) Role of AMPK and AMPK-related family in the regulation of metabolism in physiological and pathophysiological conditions.
Leptin controls body energy metabolism by reciprocally regulating AMP kinase in the hypothalamus and skeletal muscle
Leptin activates AMP kinase (AMPK) in skeletal muscle directly at the muscle level and indirectly through the hypothalamic-sympathetic nervous system. Leptin also inhibits food intake by suppressing AMPK activity in the hypothalamus. Reciprocal regualtion of AMPK activity in the hypothalamus and skeletal muscle is necessary for the leptin's effect on energy metabolism. We are intensively studying the molecular mechanism for the reciprocal regulation of AMPK activity in the hypothalamus and skeletal muscle.
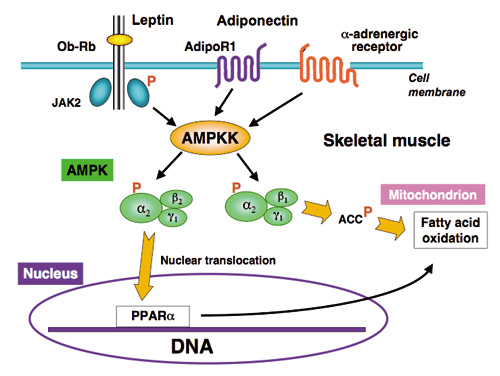
Regulatory role of AMPK in fatty acid oxidation in skeletal muscle
Leptin, adiponectin and a-adrenergic agonist activate a2AMPK in skeletal muscle via AMPKK. Activated a2AMPK containing the b2 subunit rapidly translocates the nucleus, where it induces PPAR a gene transcription. In contrast, a2AMPK containing the b1 subunit is retained in the cytoplasm, where it phosphorylates acetyl-CoA carboxylase (ACC) and thereby stimulates fatty acid oxidation.
Leptin inhibits food intake by suppression of AMPK activity in ARH-PVH axis
Arcuate hypothalamus (ARH) expresses NPY/AGRP and a-MSH neruons. Leptin inhibits NPY/AGRP neurons by decreasing AMPK activity and thereby activates melanocortin 4 receptor (MC4R) in the PVH. Activated MC4R further decreases AMPK activity in the PVH, leading to leptin-induced anorexia. Recently, we found that AMPK in the PVH regulates food preference as well as calorie intake.
Staff
 |
Professor:
MINOKOSHI, Yasuhiko, MD, PhD
1983 Graduated from Ehime University School of Medicine. 1987 Completed the doctoral course in Science in Ehime University. 1987 Research Associate in Ehime University. 1993 Lecturer in Ehime University School of Medicine. 1997 Associate Professor in Ehime University School of Medicine. 2003 Lecturer in Harvard Medical School. 2003 Professor, NIPS.
Speciality: Endocrinology and metabolism |
 |
Assistant Professor:
SHIUCHI, Tetsuya, PhD
1997 Graduated from Faculty of Integrated Arts and Sciences, The University of Tokushima. 1999 Completed the master course in Graduate School of Human and Natural Environment Sciences, The University of Tokushima. 2003 Completed the doctoral course in Ehime University School of Medicine. 2003 Research Associate, Ehime University School of Medicine. 2004 Assistant Professor, NIPS.
Speciality: Endocrinology and metabolism, Biochemical physiology of exercise |
 |
Assistant Professor:
OKAMOTO, Shiki, VMD, PhD
1996 Graduated from Faculty of Veterinary Medicine, Hokkaido University. 2000 Completed the doctoral course in Veterinary Medicine in Hokkaido University. 2000 JSPS Research Fellow. 2001 Researcher in Tokyo Metropolitan Institute of Medical Science. 2004 Assistant Professor, NIPS.
Speciality: Neuroimmunology, Stem cell biology |
 |
Postdoctoral Fellow:
LEE, Suni, PhD
1997 Graduated Tokyo University of Agriculture and Technology. 1999 Completed the master course in Graduate School of Tokyo University of Agriculture and Technology. 2002 Completed the PhD course in United Graduate School of Agricultural Sciences, Tokyo University of Agriculture and Technology. 2002 Postdoctoral fellow in Dept. Neurosciences, Case Western Reserve University (OH, US). 2003 Postdoctoral fellow in Dept. Neurological surgery, University of Miami (FL, US). 2005 Postdoctoral fellow, NIPS.
Speciality: Neurocellbiology, Neurosciences |
|