DEPARTMENT OF DEVELOPMENTAL PHYSIOLOGY
Outline
Department of Developmental Physiology was founded in 2003 with 3 full-staff divisions and 1 adjunct division, for the purpose of clarifying the physiological mechanism of mental and physical development of human beings. One of the divisions was transferred from the previous Higher Brain Function Project, Department of Integrative Physiology, headed by Prof. Tadashi Isa. This division (Division of Behavioral Development) studies the development and post-injury recovery of the neural systems controlling the eye and hand movements. Prof. Junichi Nabekura was elected as a chair of the Division of Homeostatic Development and has initiated the activity of the division since 2003. This division studies the development of synapses in the central nervous system and remodeling of neural circuits after the brain injury by using electrophysiological techniques and imaging with multi-photon microscopes. Prof. Yasuhiko Minokoshi was elected as the chair of Division of Reproductive/Endocrine Development, and started researches on neural control of metabolism, especially focused on the function of the hypothalamus, to obtain better understanding of the molecular mechanisms of pathophysiology of obesity and diabetis mellitus. Division of Adaptation Development, an adjunct division, had been chaired by Prof. Toshihiko Yada of Jichi Medical University, whose is a specialist on the energy and glucose homeostasis, since 2009.
We are investigating the neural systems controlling saccadic eye movements and grasping hand movements. We analyze the structure and function of local circuits and large-scaled networks involved in these motor systems. We are also interested in plastic compensatory mechanism following lesion of related structures as detailed below;
1. Saccadic eye movements
(1)Electrophysiological analysis of local circuits of the superior colliculus (SC), a pivotal midbrain center for saccade control, by using in vitro slice preparation.
(2)Analysis of local circuit of SC and large scaled network involving the cerebral cortex for saccade generation in anesthetized animals (rodents and non-human primates).
(3)Molecular mechanism of saccade generator circuits by using genetically manipulated mice.
(4)Analysis of dynamic properties of saccade-related circuits by applying electrophysiological and pharmacological techniques in awake behaving non-human primates.
(5)To clarify the neural mechanism of saccade control and visual awareness in "blindsight" patients, we are analyzing the saccadic behaviors and neuronal activities in macaque monkeys with unilateral lesion of the primary visual cortex (V1), as an animal model of "blindsight".
2. Dexterous hand movements
We clarified the existence of oligosynaptic (indirect) pathway from the primary motor cortex to hand motoneurons mediated by interneurons in the cervical spinal cord. Moreover, we observed the behavior of the monkeys in which direct cortico-motoneuronal connections are transected while the indirect pathway remained intact and found that the monkeys can perform precision grip after 1-3 months of recovery period. To explore the basic mechanism involved in the compensatory mechanisms, we use multidisciplinary approaches including electrophysiology, non-invasive brain imaging with positron emission tomography (PET) and analysis of gene expression by DNA microarray and in-situ hybridization.
Recently we start to approach the analysis of neural systems controlling movements by the manipulation of the function. One is the Brain-machine Interface (BMI). This technology with decoding neural activity would be useful for the analysis of neural system. The other is the gene transfer using virus vector. For example, the optogenetics is a high-powered tool to discriminate the neurons with particular function or connections.
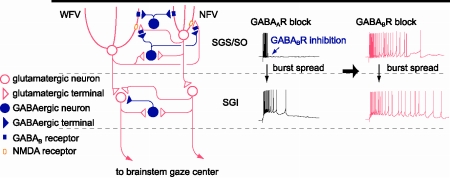
Figure 1. Schematic of local circuit underlying GABABR-mediated regulation of bursts in the SC. Postsynaptic GABABRs expressed both in NFV and WFV cells and presynaptic GABABRs located on gluamatergic synaptic terminals in the SGS are activated by synaptically released GABA during bursts of SGS GABAergic neurons. Hyperpolarization in NFV cells, shunting inhibition in WFV cells, and reduction of glutamate release may contribute to the limitation of burst duration. When GABABRs are blocked, burst duration in the SGS may be prolonged in an NMDAR-dependent manner, and then, the prolonged burst may spread to the SGI. Thus the burst duration of the SGI may also be prolonged.
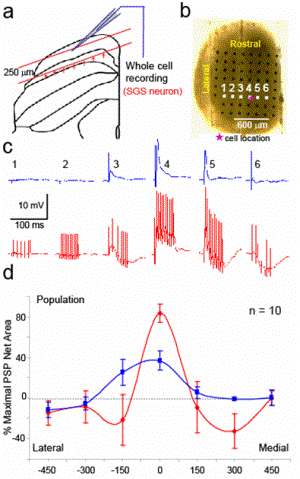
Figure 2. (a) The schematic drawing indicates the cutting plane of horizontal slice of superficial SC with arrangement of stimulating and recording system from the coronal view. The red arrows show directions of electrical stimulation that were applied. (b) Top view of the horizontal slice of superficial SC overlying an electrode array. The pink asterisk indicates the location of recorded neuron. Electrical stimulationwas applied from each of six electrodes in the white square while recording from the neuron. (c) The evoked EPSPs or IPSPs from the stimulation at electrode number 1-6 in figure b are shown for single pulse (top row blue traces) and 200 Hz (bottom row red traces) stimulation. (d) The integration of EPSP (positive value) and IPSP (negative value) from the data in panel c during 0-50 ms after stimulation onset were summarized and plotted on the distance axis that the closest electrode to the cell was set to be zero. Percent of maximal responses were plotted on the distance between the stimulating electrode to the cell closest electrode forsingle pulse (blue) and 11 pulses/ 200 Hz (red).
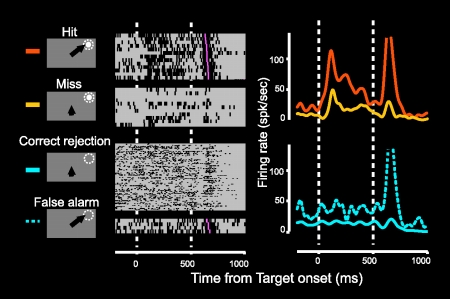
Figure 3. Neuronal activities in the SC ipsilateral to V1 lesion correlated to the monkey's performance. When the monkey succeed to detect them ('Hit' trial), this neuron exhibited clear visual responses to the visual stimuli presented in the visual field affected by the lesion, while when the monkey failed to detect them ('Miss' trial), these responses attenuated although the same visual stimuli were presented.
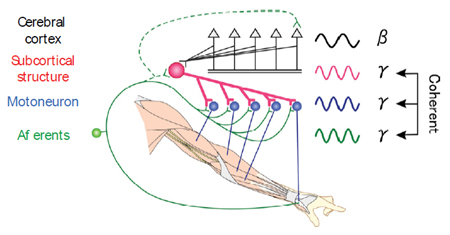
Figure 4. After spinal cord injury, a subcortical oscillator commonly recruits hand/arm muscles, via remaining pathways such as reticulospinal and/or propriospinal tracts, independent of cortical oscillation, and contributes to functional recovery.
Staff
ISA, Tadashi, MD, PhD
1985 Graduated from University of Tokyo, Faculty of Medicine. 1989 Completed the doctoral course in Science in University of Tokyo. 1989 Research Associate in University of Tokyo. 1993 Lecturer in Gunma University, School of Medicine. 1996 Professor, NIPS.
Speciality: Neurophysiology
YOSHIDA, Masatoshi, PhD
1992 Graduated from University of Tokyo, Department of Pharmaceutical Sciences. 1996 Predoctoral Fellow at NIPS and University of Tokyo. 2003 Obtained PhD from University of Tokyo. 2003 Postdoctoral Fellow at University of Tokyo. 2003 Research Associate, NIPS.
Speciality: Cognitive Neuroscience and Neurophysiology
KANEDA, Katsuyuki, PhD
1994 Graduated from Kyoto University, Department of Pharmaceutical Sciences. 1999 Completed the doctoral course in Graduate School of Pharmaceutical Sciences, Kyoto University. 1999 Postdoctoral Fellow in Tokyo Metropolitan Institute for Neuroscience. 2003 Postdoctoral Fellow in University of Tennessee. 2005 Research Associate, NIPS.
Speciality: Neurophysiology and Neuropharmacology
IKEDA, Takuro, PhD
1998 Graduated from Tokyo University, Faculty of Science. 2004 Completed the doctoral course in University of Tokyo, Faculty of Medicine. 2004 Postdoctoral Fellow at Tamagawa University. 2005 Postdoctral Fellow at NIPS. 2010, Research Associate, NIPS.
Speciality: Neurophysiology and Cognitive Neuroscience
KINOSHITA, Masaharu, PhD
1992 Graduated from University of Tsukuba, College of Biological Sciences. 1998 Completed the doctoral course in University of Tsukuba, Faculty of Medicine. 1998 Postdoctral Fellow at NIPS, 2001 Postdoctral Fellow at Rockefeller Univ., 2010 Research Associate, NIPS.
Speciality: Neurophysiology
MARUYAMA, Megumi, PhD
1998 Graduated from Osaka University, Faculty of Medicine. 2002 Research Associate, Shimane University, Faculty of Medicine. 2007 Obtained PhD from Shimane University. 2009 Research Fellow, National Institute for Basic Biology. 2010 Research Associate, NIPS.
Speciality: Neurophysiology and Environmental Physiology
NISHIMURA, Yukio, PhD
1995 Graduated from Nihon University, Faculty of Humanity and Science. 1998 Completed the master course of Graduate school in Yokohama National University, faculty of Education. 2003 Completed the doctoral course in University of Chiba, faculty of Medicine. 2003 Postdoctral Fellow, NIPS. 2007 Visiting Scientist in University of Washington. 2009 Researcher, PRESTO-JST.
Speciality: Neuroscience
WATANABE, Hidenori, PhD
1997 Graduated from Tokyo Univ. of Science, Dept. of Physics. 2002 Completed the doctoral course in The Univ. of Tokyo, S. of Engineering. Research Fellow, Tamagawa Univ.. 2003 JSPS Research Fellow. 2005 Research Fellow, Tamagawa Univ.
Speciality: Neuroscience, Information systems engineering
SAKATANI, Tomoya, PhD
1997 Graduated from the University of Tokyo, Faculty of Science. 2004 Obtained PhD from the Graduate University for Advanced Studies (SOKENDAI). 2004 Postdoctral Fellow at NIPS. 2005 Visiting Fellow at the University of Oxford. 2006 Postdoctral Fellow at NIPS.
Speciality: Neuroscience
KATO, Rikako, PhD
1997 Graduated from Ibaraki University, Faculty of Science. 2003 Completed the doctoral course in Tsukuba University, Faculty of Medicine. 2003 Postdoctral Fellow, NIPS. 2003 Postdoctral Fellow, College de France. 2005 Postdoctral Fellow, NIPS. 2010 Postdoctral Fellow, NIPS.
Speciality: Neuroscience
UMEDA, Tatsuya, PhD
1998 Graduated from University of Tokyo, Faculty of Science. 2004 Completed the doctoral course in Tokyo Medical and Dental University, Faculty of Medicine. 2005 Postdoctral Fellow at Tokyo Medical and Dental University, 2007 Postdoctral Fellow, NIPS.
Speciality: Neurophysiology
IMAZU, Sugiko
2001 Graduated from College of Liberal Arts, International Christian University. 2003 Completed the master's course in Graduate School of Informatics, Kyoto University. 2005 Editor, Kubapro. 2007 Science Communicator, National Museum of Emerging Science and Technology. 2009 Research fellow, NIPS.
PHONGPHANPHANEE, Penphimon, PhD
1999 Graduated from Faculty of Pharmaceutical Sciences, Chulalongkorn University, Thailand. 2008 Completed the doctoral course in the Graduate University for Advanced Studies(SOKENDAI). 2009 Postdoctoral Fellow in NIPS.
Speciality: Neurophysiology
In the last stage of neural development, a massive re-arrangement of neuronal circuits takes place. This is associated with an alteration of functional neural circuits, resulting in the changes in various brain functions (e.g. behavior, sensory function and biological rhythm). Our research aim is to understand the developmental re-arrangement of brain function at neuronal circuits and synaptic levels by using various approaches, e.g. in vivo imaging with advanced two photon microscopy, electrophysiology and molecular biological techniques. We also focus on the re-appearance of immature characteristics at the molecular, synapse and circuit levels after acute brain damage.
To achieve cutting edge technique for in vivo imaging with two photon microscopy, we have improved the laser light path and the cranial attachment for imaging. We succeeded in achieving a distinct imaging of various fine structures through entire layers of mouse cortex.
(1) By employing this technique, we attempt to elucidate the mobility of neuronal spines and various glia during development and after neuronal injury. We visualized the microglia surveillance for synapses in in vivo. In the intact brain, microglia attached onto the boutons, presynaptic structures, and dendritic spines, postsynaptic structures, for 5 min every 1 hour, exactly. In the damaged brain, this contact became much prolonged to more than 1 hour in duration. Occasionally, the damaged synapses were eliminated after the prolonged contact. Microglia seemed to disconnect the severely damaged neuronal circuits. In addition, the rate of synapse turnover increased at the hemisphere contralateral to damaged somatosensory cortex during limited period after the infarction, resulting in generating new circuits compensating the loss of function. In addition, we also focus on the remodeling of neuronal circuits in case of chronic pain model and GABAergic migration in the immature cortex.
(2) In immature animals, GABA induces neuronal depolarization and often acts as an excitatory substance. Intracellular CI- concentration decreases during development, resulted from developmental switch of intracellular CI- regulators, e.g. K+ CI- cotransporter2 (KCC2) and Na+, K+, CI- cotransporter 1 (NKCC1). Phosphorylation of KCC2 induces its clustering at the lipid raftand apprears its function. Neuronal damage induced dephosphorylation of KCC2, resulting in an increase in the intracellular Cl- concentration and switching GABA response from hyperpolarization to depolarization. We also study functional relevance of GABA excitation in Gn-Rh neuron, which expressed low KCC2 even in the mature, by employing several gene-manipulated mice.
(3) We reported a new form of synapse development. Transmitters to the lateral olive neurons, auditory relay neurons, switch from GABA in the immature to glycine in the mature. The transmitter switch proceeds at single synaptic terminal. This could be one of unique synaptic plasticity in developing neural circuits. We now focus on elucidating underlying mechanisms, e.g. related trophic factors.
(4) we are interested in studying the modulatory action of various nave and natural bioactive substances, e.g., TRH on the synaptic transmission and neuronal excitability by performing patch clamp recording on slice and from acutely dissociated neuron.
Staff
NABEKURA, Junichi, MD, PhD
1980 Graduated from Kyushu University, School of Medicine. 1986 Completed the doctoral course in Medical Sciences, Kyushu University. 1986 Research Fellow, Washington University. 1991 Assistant Professor, Department of Neurophysiology, School of Medicine, Tohoku University. 1993 Associate Professor, Department of Physiology, School of Medicine, Akita University. 1995 Associate Professor, Kyushu University, Graduate School of Medical Sciences. 2003 Professor, NIPS.
Speciality: Neuroscience
ISHIBASHI, Hitoshi, PhD
1988 Graduated from Kyushu University, Faculty of Pharmaceutical Sciences. 1990 Completed the master course in Pharmaceutical Sciences, Kyushu University. 1996 Completed the doctoral course in Medical Sciences, Kyushu University. 1998 Assistant Professor, Faculty of Pharmaceutical Sciences, Kumamoto University. 2000 Assistant Professor, Graduate School of Medical Sciences, Kyushu University. 2007 Associate Professor, NIPS.
Speciality: Neuroscience
KATO, Go, MD, PhD
1998 Graduated from Kyushu University, School of Medicine. 2005 Completed the doctoral course in Medical Sciences, Kyushu University. 2005 Postdoctoral Fellow, Beth Israel Deaconess Medical Center, Harvard Medical School. 2007 Instructor, Beth Israel Deaconess Medical Center, 2008 Assistant Professor, Department of Orthopedic Surgery, School of Medicine, Kyushu University. 2009 Resident, Department of Orthopedic Surgery, Fukuoka City Hospital. 2010 Assistant Professor, NIPS.
Speciality: Neuroscience
WATANABE, Miho, PhD
1996 Graduated from Waseda University, Faculty of Human Sciences. 1998 Completed the master course in Human Sciences, Waseda University. 2004 Completed the doctoral course in Medicine, Nippon Medical School. 2004 Research Fellow, NIPS. 2006 Research associate, NIPS.
Speciality: Neuroscience.
ETO, Kei, PhD
2004 Graduated from University of Shizuoka, Faculty of Pharmaceutical Sciences. 2006 Completed the master course in Pharmaceutical Sciences, Kyushu University. 2009 Completed the doctoral course in Pharmaceutical Sciences, Kyushu University. 2007 JST Research Fellow.
Speciality: Neuroscience
KIM, Sun Kwang, OMD, PhD
2002 Graduated from Kyung Hee University, College of Oriental Medicine. 2008 Completed the doctoral course in Oriental Medicine, Kyung Hee University. 2008 Research fellow, Acupuncture & Meridian Science Research Center, Kyung Hee University. 2008 JSPS fellow.
Speciality: Neuroscience
The animal body has an integrated-regulatory system for "homeostasis" that maintains a normal, constant-internal state by responding to changes in both the external and internal environments. Within the central nervous system, the hypothalamus is a crucial center that regulates the homeostatic activities by integrating autonomic nervous system, endocrine system and immune function. In this division, we are extensively investigating the role of hypothalamus in body energy balance in mammals. These studies are now important for better understanding the molecular mechanisms behind pathophysiology of obesity and diabetes mellitus. The main subjects of our current research are as follows:
(1) Molecular mechanism of the hypothalamic regulation of food intake.
(2) Regulatory role of the hypothalamic-sympathetic nervous system in glucose and lipid metabolism.
(3) Signaling pathway for metabolic action of leptin and adipokines in peripheral tissues.
(4) Role of AMPK and AMPK-related family in the regulation of metabolism in physiological and pathophysiological conditions.
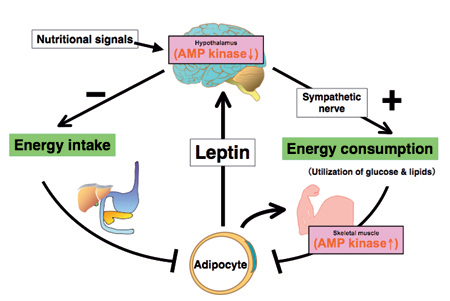
Leptin controls body energy metabolism by reciprocally regulating AMP kinase in the hypothalamus and skeletal muscle
Leptin activates AMP kinase (AMPK) in skeletal muscle directly at the muscle level and indirectly through the hypothalamic-sympathetic nervous system. Leptin also inhibits food intake by suppressing AMPK activity in the hypothalamus. Reciprocal regualtion of AMPK activity in the hypothalamus and skeletal muscle is necessary for the leptin's effect on energy metabolism. We are intensively studying the molecular mechanism for the reciprocal regulation of AMPK activity in the hypothalamus and skeletal muscle.
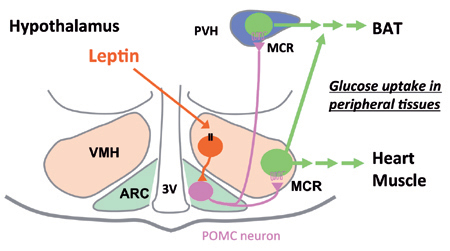
Regulatory role of the hypothalamic nuclei in glucose metabolism in peripheral tissues in response to leptin
Leptin activates POMC neurons in arcuate hypothalamus (ARC) via VMH neurons, thereby stimulating melanocortin receptor (MCR) in VMH and PVH neurons. Activation of MCR in VMH stimualtes gluocse uptake in BAT, heart and skeletal muscle, while MCR in PVH stimulates glucose uptake in BAT preferentially.
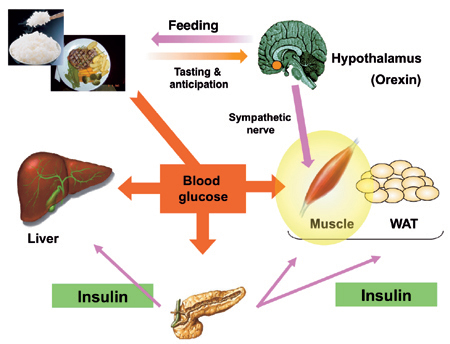
Stimulatory role of orexin in glucose metabolism in skeletal muscle
Orexins are hypothalamic neuropeptides that play important roles in the regulation of sleep/wakefulness, feeding and motivated behavior. We found that orexin neurons activate in reponse to tasting and anticipation of feeding, and then activate VMH neurons. Actiavated VMH neurons stimulate glucose utilizaiton in skeletal muscle preferentially, thereby preventing diet-induecd hyperglycemia.
Staff
MINOKOSHI, Yasuhiko, MD, PhD
1983 Graduated from Ehime University School of Medicine. 1987 Completed the doctoral course in Science in Ehime University. 1987 Research Associate in Ehime University. 1993 Lecturer in Ehime University School of Medicine. 1997 Associate Professor in Ehime University School of Medicine. 2003 Lecturer in Harvard Medical School. 2003 Professor, NIPS.
Specialty: Endocrinology and metabolism
SHIUCHI, Tetsuya, PhD
1997 Graduated from Faculty of Integrated Arts and Sciences, The University of Tokushima. 1999 Completed the master course in Graduate School of Human and Natural Environment Sciences, The University of Tokushima. 2003 Completed the doctoral course in Ehime University School of Medicine. 2003 Research Associate, Ehime University School of Medicine. 2004 Assistant Professor, NIPS.
Specialty: Endocrinology and metabolism, Biochemical physiology of exercise
OKAMOTO, Shiki, VMD, PhD
1996 Graduated from Faculty of Veterinary Medicine, Hokkaido University. 2000 Completed the doctoral course in Veterinary Medicine in Hokkaido University. 2000 JSPS Research Fellow. 2001 Researcher in Tokyo Metropolitan Institute of Medical Science. 2004 Assistant Professor, NIPS.
Specialty: Neuroimmunology, Stem cell biology
INAGAKI-OHARA, Kyoko, PhD
1988 Graduated from Faculty of Applied Biological Science, Hiroshima University (MS). 1997 Completed the PhD course in Graduated School of Medicine, Nagoya University. 1997 Research Associate in Medical School of Northwestern University (IL, US), Kyushu University, 2003 Assistant Professor Dept. Medicine, University of Miyazaki, Project Assistant Professor University of the Ryukyu.
Specialty: Mucosal Immunity
TODA, Chitoku, PhD
2006 Graduated from Faculty of Veterinary Medicine, Hokkaido University. 2009 Completed the doctoral course in The Graduate University for Advanced Studies (SOKENDAI). 2009 Postdoctoral fellow, NIPS.
Specialty: Endocrinology and metabolism
Regulation of insulin release: 1) Elucidating mechanisms by which ghrelin inhibits insulin release, and establishing the basis for treating type 2 diabetes with ghrelin blockade that promotes insulin release. 2) Elucidating the role of Kv channels of islet b-cells in regulation of insulin release.
Regulation of feeding: 1) Elucidating feeding-regulating neural pathway in the hypothalamus, particularly that from the 1st order center Arcuate nucleus to the integrative center Paraventricular nucleus. 2) Elucidating mechanisms how novel anorectic peptide Nesfatin-1 and classical peptide Oxytocin regulate feeding and exploring their (patho)physiological roles and therapeutic potential. 3) Interplay of brain and peripheral organs in regulation of feeding and body's homeostasis.
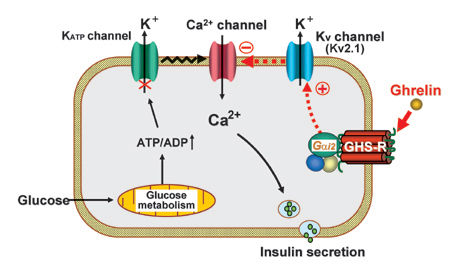
Fig 1. Mechanism for inhibition of insulin secretion by ghrelin. (Dezaki K., Yada T. et al.: Diabetes 56: 2319-2327, 2007; Dezaki K., Sone H., Yada T.: Pharmacol. Ther. 118: 239-249, 2008)
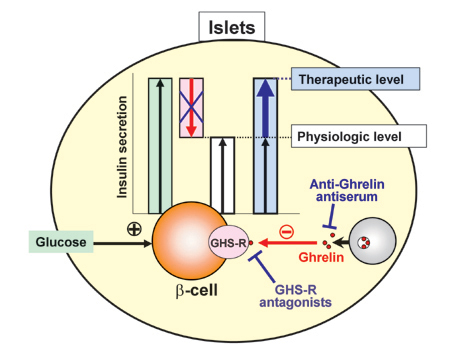
Fig 2. Endogenous ghrelin in islets inhibits and its blockade promotes insulin secretion. (Dezaki K., Yada T. et al.: Diabetes 53: 3142-3151, 2004; Diabetes 55: 3486-3493, 2006.; Yada T., Dezaki K., et al. Curr. Diab. Rev. 4: 18-23, 2008.)
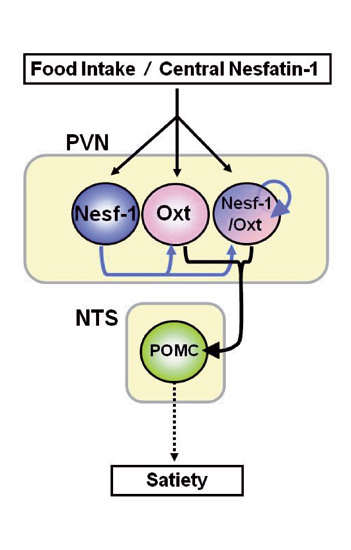
Fig 3. Nesfatin-1-operative PVN oxytocinergic signaling to NTS POMC neurons causes melanocortin-dependent satiety
Central Nesf-1 or food intake activates Nesf-1 and Oxt neurons in PVN. In PVN, endogenous Nesf-1 serves as a paracrine/autocrine or local neuronal stimulator of Oxt neurons. PVN oxytocinergic signaling to NTS activates POMC neurons, inducing melanocortin-dependent satiety. (Maejima Y., Yada T. et al.: Cell Metabolism 10: 355-365, 2009)
Staff
YADA, Toshihiko, PhD
1975 Graduated from Hokkaido University, School of Engeneering. 1983 Completed the doctoral course at Kyoto University, Graduate School of Medicine. 1983-1987 Research Associate, Tokyo Medical and Dental University, School of Medicine, Department of Physiology. 1986-1987 Postdoctoral Associate, Cornell University, College of Veterinary Medicine, Department of Pharmacology, USA. 1987-2000 Associate Professor, Kagoshima University, School of Medicine, Department of Physiology. 1996-2000 Adjunct Associate Professor, NIPS, Division of Intracellular Metabolism. 2000- Professor, Department of Physiology, Division of Integrative Physiology, Jichi Medical University, School of Medicine. 2009- Adjunct Professor, NIPS, Department of Developmental Physiology, Division of Adaptation Development.
























