DEPARTMENT OF INFORMATION PHYSIOLOGY
Outline
Molecular approach has been very successful in identifying and elucidating functional elements and their functions, and imaging techniques have provided a large amount of information of the functional localization of the cerebral cortex and other brain structures. It remains largely unknown, however, how information is processed in the neuronal networks, which connect the microscopic and macroscopic levels of the brain. In the Department of Information Physiology, both of top-down and bottom-up approaches are taken to investigate the mechanism of information processing of the brain.
The main purpose of this division is to study the neural mechanisms of visual perception. The human visual system is a complicated parallel and distributed system where several neural structures play different roles, but are still able to generate a unified and integrated precept of the outer world. This system also has sophisticated mechanisms that enable reconstruction of three-dimensional structures from two-dimensional retinal images. To understand the neural substrates of these abilities in our visual system, we are recording neuronal activities from the primary visual cortex and extrastriate visual areas. We are analyzing the stimulus selectivity of neurons to determine the representation of various kinds of visual features, suchas color, motion, shape and depth. We are also studying the dynamics of visual information processing in the cortex by analyzing the temporal pattern of neural activities. In addition, to explore the ways in which various visual features contribute to visual perception, psychophysical experiments are conducted in this laboratory.
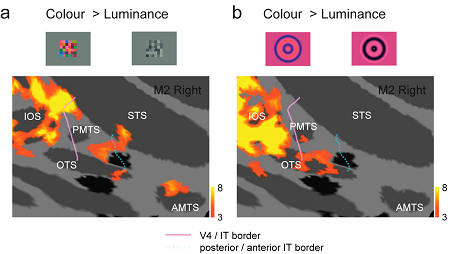
Inferior temporal (IT) cortex in monkey plays an important role in color processing. However, little is known about how colour information is distributed in these cortical regions. We explored the distribution of colour-selective activity in alert macaque monkeys using functional magnetic resonance imaging (fMRI) with two types of stimuli: a multicoloured ('Mondrian') pattern (a) and a colour grating (b). We found that colour-selective activities in the IT cortex are not distributed uniformly, but are localized in discrete regions, each extending several millimetres in the anterior or posterior part of the IT cortex. The colour-selective activation in the anterior IT was observed only with the Mondrian stimuli, whereas the colour-selective activation in the posterior IT was observed with both the Mondrian and grating stimuli, with little overlap. These findings suggest that there are multiple subregions with differing stimulus selectivities distributed in the IT cortex.
Staff
KOMATSU, Hidehiko, PhD
1982 Completed the doctoral course in Osaka University. 1982-1988 Hirosaki University. 1985-1988 National Eye Institute, U.S.A. 1988-1995 Electrotechnical Laboratory. 1995 Professor, NIPS.
Speciality: Neurophysiology
ITO, Minami, PhD
1989 Completed the doctoral course in Osaka University. 1989-1994 Riken Institute. 1994-1998 Rockefeller University. 1998 Associate Professor, NIPS.
Speciality: Neurophysiology
GODA, Naokazu, PhD
1998 Completed the doctoral course in Kyoto University. 1998-2003 ATR. 2003 Assistant Professor, NIPS.
Speciality: Visual Psychophysics
Our main interest lies in elucidation of the mechanism of transduction and integration of neural information in the nervous system. More specifically, we are trying to understand the basic properties of neural information processing between neurons or among a group of neurons constituting a local network. We are also interested in the pathophysiological mechanism how a single gene mutation leads to a symptom (such as ataxia, epilepsy and learning and memory deficits), particularly in Ca2+ channel mutant and Ca2+/calmodulin-dependent protein kinase IIa mutant mice. Additionally, we have recently started to make a computational approach, incorporating computer-based neurons into brain slice measurements (dynamic clamp), together with computational simulation of network functions. The following are currently ongoing major projects.
(1) Studies of neurological disorders caused by calcium channel mutations. Mutations of the voltage-gated calcium channels are associated with neurological disorders of human and mice, which include cerebellar ataxia and some forms of seizure disorders. We study the relation how a single mutation causes neurological manifestations, mainly using brain slice preparations.
Recently, we identified a dramatic impairment in the neural circuit of feedforward inhibition in the thalamocortical projection in epileptic calcium channel mutant mice tottering (Fig. 1).
(2) Analysis of inhibitory synaptic circuits in the spinal dorsal horn and its plastic change. We investigate the modulatory mechanism of spinal nociceptive transmission by using in vivo patch-clamp recording techniques from spinal dorsal horn and locus coeruleus neurons (Fig. 2). The underlying mechanism for the development of chronic pain is also a topic of our current interest.
(3) Transmitter diffusion-mediated crosstalk between heterologous neurons. We previously reported that the excitatory transmitter glutamate diffused from climbing fibers (CFs) [projection to cerebellar Purkinje cells (PCs) from the inferior olive in the brain stem] presynaptically inhibited the GABAergic information flow from basket cells to the same PCs. Recently, we found that glutamate transporters (EAAT4/GLAST/GLT-1) take unique part in determining the degree of CF-induced inhibition by influencing the glutamate concentration in the route of its intersynaptic diffusion (Fig. 3).
(4) Role of protein phosphorylation in neuronal functions. Ca2+/calmodulin-dependent protein kinase IIa (CaMKIIa) is an enzyme that adds phosphates to a variety of protein substrates to modify their functions. It is believed to be an essential mediator for activity-dependent synaptic plasticity and memory functions. We recently generated a kinase-dead CaMKIIa (K42R) knock-in mouse, and found that both hippocampal synaptic plasticity and behavioral learning are severely impaired in this mutant mouse (Fig. 4). We are now trying to analyze further molecular mechanisms for such impairments and examine other aspects of learning and memory in the K42R knock-in mouse.
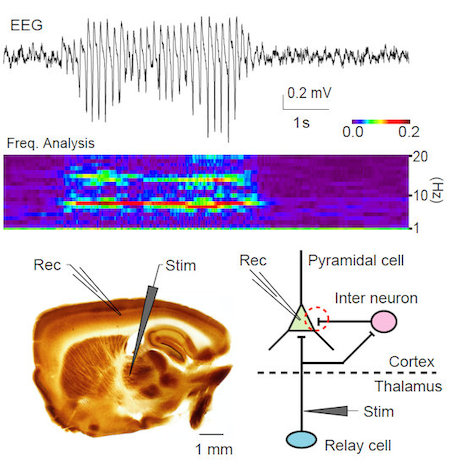
Figure 1. EEG of a tottering mouse during absence seizure (top). Frequency analysis of this EEG shows enhanced spectrum around 6 - 7 Hz during the absence seizure (middle). A brain slice preparation in which the connection between the thalamus and the cerebral cortex is preserved (bottom left). The disynaptic inhibitory input to cortical cells from the thalamus was reduced in tottering mice (bottom right, red dashed circle).
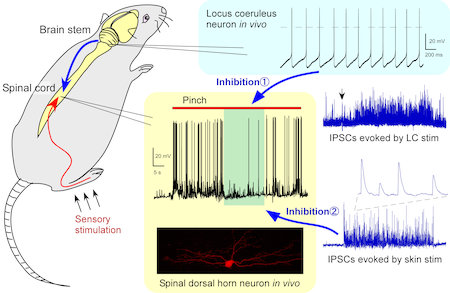
Figure 2. In vivo patch-clamp analysis of inhibitory mechanisms of nociceptive transmission. Activation of locus coeruleus neurons resulted in an inhibition of spinal nociceptive transmission by enhancement of inhibitory synaptic responses. Cutaneous innocuous stimuli also elicited a barrage of inhibitory synaptic responses in the spinal dorsal horn.
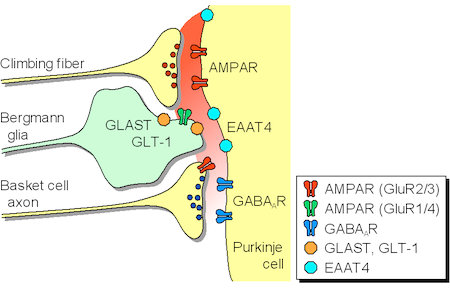
Figure 3. Glutamate released from the climbing fibers diffuses from the synaptic cleft and activates presynaptic AMPA receptors (GluR2/3) of the basket cell terminals, leading to inhibition of GABA release from the basket cells. Extrasynaptic glutamate concentration is controlled by glutamate transporters (EAAT4/GLAST/GLT-1).
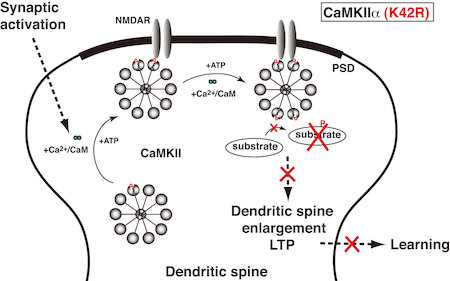
Figure 4. Kinase activity of CaMKIIa is essential for hippocampal synaptic plasticity and behavioral learning. In the kinase-dead CaMKIIa (K42R) knock-in mouse, synaptic activation induces binding of Ca2+/CaM to CaMKIIa and its postsynaptic translocation, but CaMKIIa (K42R) cannot phosphorylate substrate proteins, and therefore, dendritic spine enlargement, long-term potentiation and behavioral learning are all impaired.
Staff
IMOTO, Keiji, MD, PhD
Graduated from Kyoto University Faculty of Medicine. Medical Staff, National Utano Hospital. Instructor, Lecturer, and Associate Professor, Kyoto University Faculty of Medicine. Research Associate, Max-Planck-Institut für medizinische Forschung. 1995 Professor, NIPS.
Specialty: Neurophysiology
FURUE, Hidemasa, PhD
Graduated from Kyushu Institute of Technology Graduate School of Computer Science and System Engineering. Research Associate, Saga Medical School. Research Associate and Assistant Professor, Kyushu University Graduate School of Medical Sciences. 2009 Associate Professor, NIPS.
Specialty: Neurophysiology
YAMAGATA, Yoko, MD, PhD
Graduated from Kyoto University Graduate School of Medicine. Research Associate, Kyoto University Faculty of Medicine. Postdoctoral Fellow, The Rockefeller University. 1991 Assistant Professor, NIPS.
Specialty: Biochemistry, Neurochemistry
SATAKE, Shin'Ichiro, PhD
Graduated from Nagoya University Graduate School of Science. Postdoctoral Fellow of Mitsubishi Kagaku Institute of Life Sciences, Research Fellow of CREST (JST). 2002 Assistant Professor, NIPS.
Specialty: Neurophysiology
KASE, Daisuke, PhD
Graduated from The Graduate University for Advanced Studies School of Life Sciences. 2009 Postdoctoral Fellow, NIPS.
Specialty: Neurophysiology
UTA, Daisuke
Graduated from Kyushu University Graduate School of Medical Sciences. 2010 Research Fellow, NIPS.
Specialty: Neurophysiology
Division of Developmental Neurophysiology
Department of Development
Differentiation and Regeneration,
OKAZAKI INSTITUTE FOR
INTEGRATIVE BIOSCIENCE
1. Analysis of the neuronal circuits in visual cortex
Primary visual cortex is one of the best areas to study the relationship between brain functions and synapses/neural circuits, because the visual responsiveness of each neuron and the functional columnar structures are well characterized in this area. Therefore, we have analyzed the synapses and neuronal circuits in this cortical area, and clarified their basic properties. For example, we tested for fine-scale specificity of connections in rat visual cortex using cross-correlation analyses of synaptic currents evoked by laser scanning photostimulation. Recording simultaneously from adjacent layer 2/3 pyramidal cells, we found that when the cells were connected they shared inputs from individual excitatory neurons in layer 4 and layer 2/3. Thus, excitatory connections from layer 4 to layer 2/3 and within layer 2/3 form fine-scale assemblies of selectively interconnected neurons. To characterize connection properties further and elucidate the role of the fine-scale circuit in visual information processing, we are currently conducting electrophysiological analyses of neural circuits using slice preparations prepared from transgenic mice in which cells responding to particular visual stimulation can be visualized by fluorescent proteins expressed in an activity-dependent manner.
2. The activity-dependent developmental of visual responsiveness and neuronal circuits
It is known that visual function matures under the strong influence of postnatal experience. We have been examining the effect of manipulation of visual inputs on the development of synaptic connections and neuronal circuits, to unravel the synaptic mechanisms of activity-dependent maturation of cortical functions.
3. Neuronal basis of locomotion and its development
Recent molecular genetic studies suggest that the expression of transcription factors in the developing spinal cord helps determine the morphological and physiological properties of neurons. Using the zebrafish preparation, we have been examining the electrophysiological and morphological properties of neurons specified by individual or sets of transcription factors.
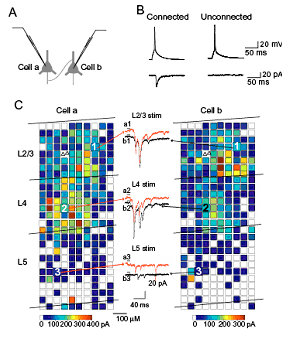
Analyses of photostimulation-evoked excitatory postsynaptic currents (EPSCs) simultaneously recorded in a pair of layer 2/3 pyramidal neurons that was synaptically connected.
For each of the two cells, reconstructions of the locations of photostimulation sites (coloured squares) relative to the locations of laminar borders and cell bodies of recorded pyramidal neurons (triangles) are shown. The colour of each square indicates the sum of amplitudes of EPSCs that were observed in response to photostimulation at that site.
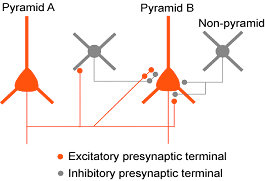
Inhibition between nearby pyramidal neurons via inhibitory synaptic terminals
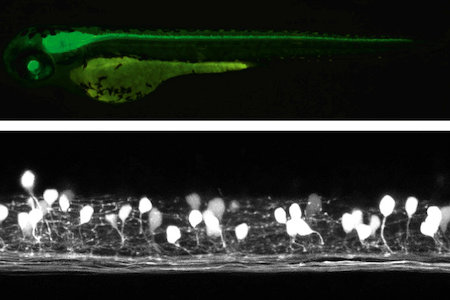
Studies with zebrafish as a model system to understand molecular mechanisms underlying development of neuronal wiring and neurophysiology of locomotion.
In the transgenic zebrafish, a class of inteneurons are easily identified by fluorescence of GFP in live animals. The upper panel is an image using a regular fluorescent micoscope. The bottom panel is an image by a confocal microscopy.
Staff
YOSHIMURA, Yumiko, PhD
1989 Graduated from Osaka Prefecture University. 1995 Completed the doctoral course, Osaka University, Faculty of Medicine. 1995 Postdoctral Fellow, Osaka Bioscience Institute. 1997 Assistant Professor, Nagoya University. 2006 Associate Professor, Nagoya University. 2009 Professor, NIPS.
Speciality: Neurophysiology
HIGASHIJIMA, Shin-ichi, PhD
1989 Graduated from University of Tokyo, Faculty of Science. 1994 Completed the doctoral course in Science, University of Tokyo. 1994 Research Associate, National Institute for Basic Biology. 1996 PREST Researcher. 1998 Research Scientist, State University of New York at Stony Brook. 2003 Associate Professor, NIPS.
Speciality: Developmental Neurobiology, Neurophysiology
MORI, Takuma, PhD
Graduated from Faculty of Science, Kyoto University. Completed the doctoral course in Science, Kyoto University. Postdoctoral fellow, Primate Research Institute, Kyoto University. Research associate, Salk Institute for Biological Studies. Assistant Professor, NIPS.
Speciality: Neurophysiology, Virology
TARUSAWA, Etsuko, PhD
2006 Graduated from School of Life Science, the Graduate University for Advanced Studies. 2006 Postdoctoral Fellow at NIPS.
Speciality: Neuroanatomy
KIMURA, Yukiko, PhD
1999 Graduated from Saitama University. 2004 Completed the doctoral course in biological sciences, the University of Tokyo. 2004 Research fellow, NIPS.
Speciality: Developmental Biology
ISHIKAWA, Ayako, PhD
2008 Completed the doctoral course in Osaka University. 2008 Research Fellow, JSPS. 2009 Postdoctoral Fellow, NIPS.
Speciality: Neurophysiology
SATOU, Chie, PhD
2005 Graduated from Waseda University. 2010 Completed the doctoral course in the Graduate University for Advanced Studies.
Specialty: Neurophysiology















