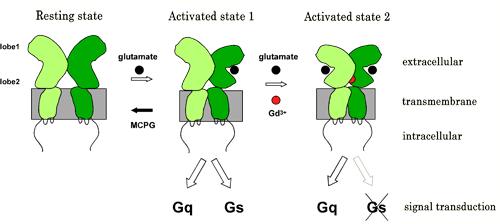
The metabotropic glutamate receptor 1alpha (mGluR1 alpha) is crucial for some forms of synaptic plasticity, by inducing various cell responses via coupling to various types of G proteins. Upon glutamate binding, an active conformation, closed-open/Active (CO/A), of the extracellular domain (ECD) is stabilized, which induces dimeric rearrangement in the intracellular domains (ICD), resulting in the initiation of downstream signalings. We have confirmed that mGluR1 functionally interacts with both Gq and Gs pathways; a combination of fluorescent indicators showed that glutamate increased intracellular Ca2+and cAMP concentration ([Ca2+]i and [cAMP]i). By contrast, Gd3+, a different type of ligand whose recognition site on mGluR1 alpha is distinct from glutamate, increased only [Ca2+]i and the concentration-activation curve was bell-shaped. Fluorescence resonance energy transfer (FRET) analysis revealed that low concentration of Gd3+ induced dimeric rearrangement of the ICD of mGluR1 alpha as like glutamate, while high concentration of Gd3+ reversed the FRET efficiency, which was consistent with bell-shaped relationship between concentration and Gq activation. These results suggested that Gd3+ induces an active and a sort of "inactivated" conformation in mGluR1alpha. The Gd3+-induced active state is considered to correspond to the closed-closed/Active conformation (CC/A), revealed by previous X-ray crystallographic studies. In conclusion, the glutamate-induced CO/A state coupled both to Gs and Gq proteins whereas Gd3+-induced CC/A preferred Gq to Gs, suggesting that mGluR1alpha serves not only as a simple On/Off switch but also as a multiple signaling path regulator.
Tateyama, M. and Kubo, Y. Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor1alpha. Proc. Natl. Acad. Sci. U.S.A. 103, 1124-1128 (2006)