Voltage-dependent proton channel currents are present in alveolar epithelium, macrophage, granulocyte, osteoclast and microglia, and play roles in producing superoxide anion (O2-) during respiratory burst for phagocytosis. However, the molecular basis underlying this channel has long been elusive. Here we identify a cDNA which shows homology to the voltage sensor domain (VSD) of voltage-gated channels. Its deduced protein consists only of S1-S4 segment, thus being named as voltage-sensor domain only protein (mVSOP). In tsA201 cells expressing mVSOP, depolarizing step induced slowly-activating outward currents accompanied by tail inward currents at repolarization. Tail currents measured under different pH conditions were reversed at voltage levels corresponding to equilibrium potentials for protons that are predicted from Nernst equation, indicating that these currents are through proton-selective channels. Imaging analysis demonstrated that pHin recovers rapidly after an acid load in mVSOP-transfected cells. VSOP-induced currents showed hallmark feature of native voltage-dependent proton channel; shift of threshold and kinetics of activation occurred dependent on the pH difference across the cell membrane. VSOP shares other properties with native voltage-dependent proton currents, including sensitivity to polyvalent cation such as Zn2+ and Cd2+ and relatively high temperature sensitivity. A neutralizing mutation of a positively charged residue in S4-like segment rendered negative shift of activation by about 50 mV, causing significant inward current. This indicates that this site is critical for the channel to be kept close at the membrane potential where the direction of the driving force for proton flow is inward, consistent with the previous view that outward rectification of voltage-dependent proton channel is based on its gating nature. Therefore VSOP constitutes the main molecular component of voltage-dependent proton channel.
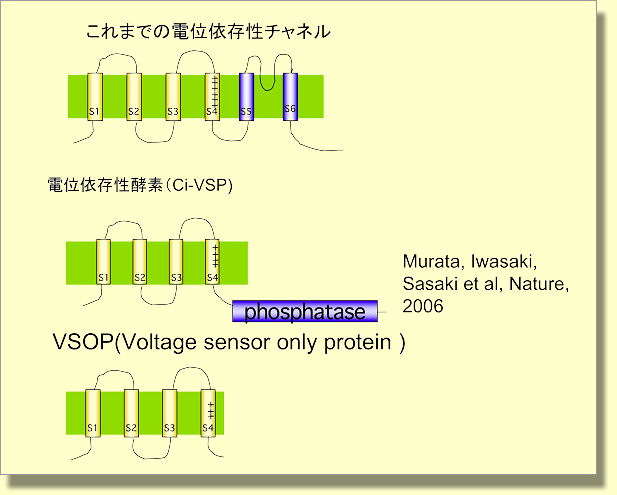
Membrane topology of three types of voltage-sensor-domain proteins. Upper: Voltage-gated Na, K, Ca channels, Middle: a voltage-regulated phosphatase, Ci-VSP (Nature, 2005; Murata et al.), Lower: VSOP
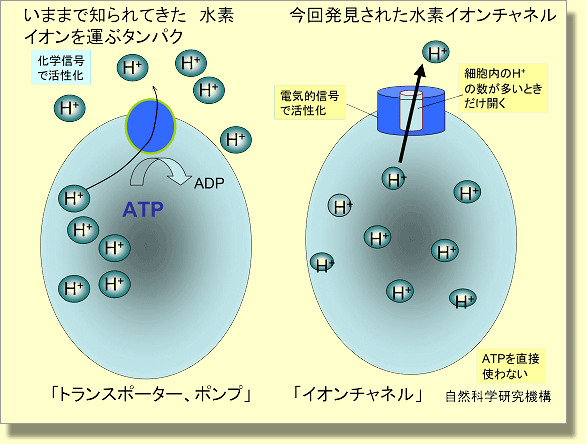
Scheme showing how voltage-gated proton channel is different from proton pump. Proton pump (left) transfers proton from the intracellular side the extra-cellular side, accompanied by ATP hydrolysis. This enables proton transfer against the proton gradient across the cell membrane. On the other hand, voltage-gated proton channel (right) has a proton pathway that is both accessible from extra-cellular side and intracellular side. Gating of this pathway is regulated both by membrane voltage and proton concentration; it is opened with a higher probability when the cell membrane is depolarized and/or the difference of pH between extra-cellular medium and intracellular medium becomes larger.
Yasushi Okamura, MD PhD
Okazaki Institute for Integrative Bioscience
National Institutes of Natural Sciences
Higashi 5-1, Myodaiji-cho, Okazaki, 444-8787, JAPAN
TEL 81-564-59-5256 FAX 81-564-59-5259