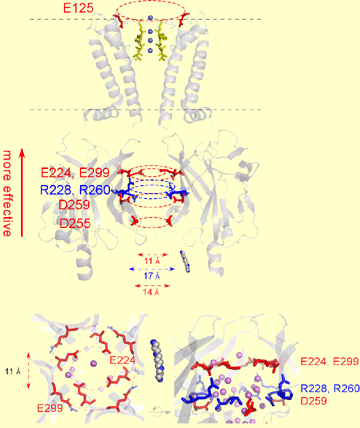
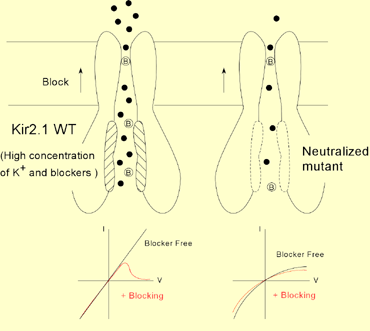
It is known that rectification of currents through the inward rectifier K+ channel (Kir) is mainly due to blockade of the outward current by cytoplasmic Mg2+ and polyamines. Analyses of the crystal structure of the cytoplasmic region of Kir2.1 have revealed the presence of both negatively (E224, D255, D259, E299) and positively (R228, R260) charged residues on the wall of the cytoplasmic pore of Kir2.1, but the detail is not known about the contribution of these charged residues, the positive charges in particular, to the inward rectification. We therefore analyzed the functional significance of these charged amino acids using single/double point mutants in order to better understand the structure-based mechanism underlying inward rectification of Kir2.1 currents. As a first step, we used two-electrode voltage clamp to examine inward rectification in systematically prepared mutants in which one or two negatively or positively charged amino acids were neutralized by substitution. We found that the intensity of the inward rectification tended to be determined by the net negative charge within the cytoplasmic pore. We then used inside-out excised patch clamp recording to analyze the effect of the mutations on blockade by intracellular blockers and on K+ permeation. We observed that a decrease in the net negative charge within the cytoplasmic pore reduced both the susceptibility of the channel to blockade by Mg2+ or spermine and the voltage-dependence of the blockade. It also reduced K+ permeation; i.e., it decreased single channel conductance, increased open-channel noise, and strengthened the intrinsic inward rectification in the total absence of cytoplasmic blockers. Taken together, these data suggest that the negatively charged cytoplasmic pore of Kir electrostatically gathers cations such as Mg2+, spermine and K+, so that the transmembrane pore is sufficiently filled with K+ ions, which enables strong voltage-dependent blockade with adequate outward K+ conductance.
Fujiwara Y, Kubo Y. Functional roles of charged amino acid residues on the wall of the cytoplasmic pore of Kir2.1. J Gen Physiol. 2006 Apr;127(4):401-19.