Seizure is a form of excessive neuronal excitation and seizure-induced neuronal damage has profound effects on the prognosis of epilepsy. In various seizure models, the inactivation of Ca2+⁄calmodulin-dependent protein kinase II (CaMKII) occurs during seizure activity preceding neuronal cell death. CaMKII is a multifunctional protein kinase enriched in the brain and involved in various ways the regulation of neuronal activity. CaMKII inactivation during seizure activity may modify neuronal cell survival after seizure. However, the mechanism for CaMKII inactivation and its consequence after seizure recovery remain to be elucidated yet. In the present study, we employed a prolonged seizure model by systemic injection of kainic acid into rats and biochemically examined the activity state of CaMKII. In status epilepticus induced by kainic acid, not only the inactivation of CaMKII in brain homogenate, but also a shift in the distribution of CaMKII protein from the soluble to particulate fraction occurred in both hippocampus and parietal cortex. The particulate CaMKII showed a large decrease in the specific activity and a concurrent large increase in the autophosphorylation ratio at Thr-286 (α) and at Thr-287 (β). In contrast, the soluble CaMKII showed normal or rather decreased specific activity and autophosphorylation ratio. After 24 h of recovery from kainic acid-induced status epilepticus, all such changes had disappeared. On the other hand, the total amount of CaMKII was decreased by 35% in hippocampus and 20% in parietal cortex, but the existing CaMKII was indistinguishable from those of controls in terms of the autonomous activity ratio, specific activity and autophosphorylation ratio. Thus, CaMKII inactivation in kainic acid-induced status epilepticus seems to be derived not from simple degradation of the enzyme, but from the formation of the autophosphorylated, inactivated and sedimentable CaMKII. Such a form of CaMKII may be important during pathological conditions in vivo in preventing excessive CaMKII activation due to Ca2+ overload.
Yamagata, Y., Imoto, K. and Obata, K. A mechanism for the inactivation of Ca2+⁄calmodulin-dependent protein kinase II during prolonged seizure activity and its consequence after the recovery from seizure activity in rats in vivo. Neuroscience 140: 981-992, 2006.
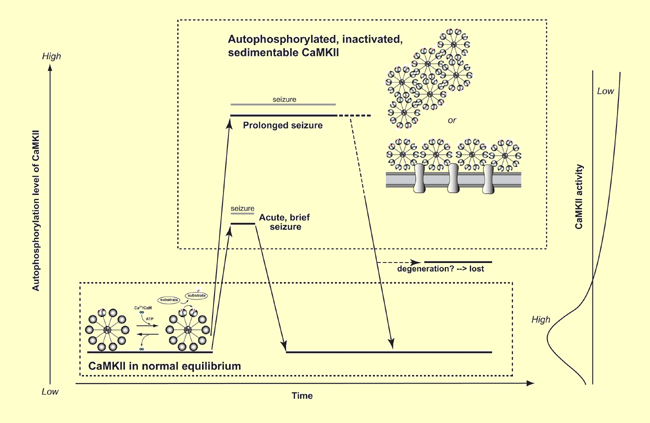
[Figure legend]
A hypothetical scheme illustrating the activity state of CaMKII during acute, brief seizure activity (Yamagata and Obata, 2004) and prolonged seizure activity (Yamagata, Imoto and Obata, 2006). In acute, brief seizure activity, autophosphorylated, inactivated and sedimentable CaMKII is completely reversible: After the termination of seizure activity, CaMKII can restore completely normal properties, and no loss of CaMKII is observed. On the other hand, in prolonged seizure activity, part of CaMKII seems to become irreversible: After complete recovery from seizure activity, part of CaMKII is lost, but the remaining CaMKII shows completely normal properties. Thus, the complete reversibility of autophosphorylated, inactivated and sedimentable CaMKII to normal equilibrium state seems to depend on the duration of seizure activity. The formation of such autophosphorylated and inactivated CaMKII during seizure activity seems to be important for trapping excess amounts of activated calmodulin and suppressing CaMKII-mediated signal transduction pathway due to Ca2+ overload during pathological conditions in vivo.