Murata Y and Okamura Y (2007) Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. Journal of Physiology 583: 875-889.
Voltage-gated ion channels are best known for their function in neurons and muscles where they are activated by changes in electrical potential difference to propagate electrical signals. All voltage-gated ion channels share a voltage sensor domain (VSD) and a pore domain. Recently a VSD-containing protein, Ci-VSP, was identified that translates electrical inputs into chemical signals but lacks a pore domain. However, it was not clear whether depolarization or hyperpolarization induces phosphatase activity of Ci-VSP. In addition, there is no explanation why there is a large gap in the voltage dependency between the charge movement of the VSD and phosphatase activities.
This paper set out to determine the relationship between the charge carried by the VSD and phosphatase activity by measuring potassium channel activities. In contrast to the original hypothesis, it was found that phosphatase is activated by depolarization, not hyperpolarization. The gap of voltage dependency between the charge movement and phosphatase activity was determined to be due to the limited dynamic range of sensitivity of GIRK2 channels to phosphoinostitides. Thus, the enzyme activity in Ci-VSP is changed gradually over a range of membrane potentials where the magnitude of ‘gating’ charges of the VSD varies.
These results suggest that Ci-VSP is similar to voltage-gated Na+, Ca2+ and K+ channels in that depolarization activates effector operation and conformational change of the VSD is translated into a downstream effector. Future research aimed at understanding the mechanism of phosphatase-voltage coupling and the structure-function relationships relevant to all voltage-gated channels.
http://www.blackwellpublishing.com/press/pressitem.asp?ref=1427
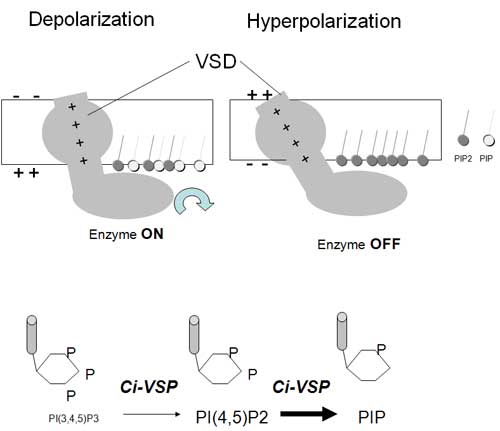
Upper panel: depolarization induces upward charge displacement of the voltage sensor and this triggers activation of phosphatase.
Lower panel: Ci-VSP dephosphorylates not only PtdIns(3,4,5)P3 but also PtdIns(4,5)P2.