Hirohide Iwasaki, Yoshimichi Murata, Youngjun Kim, Md Israil Hossain, Carolyn A. Worby, Jack E. Dixon, Thomas McCormack, Takehiko Sasaki, and Yasushi Okamura
 Phosphatidylinositol lipids play diverse physiological roles, and their concentrations are tightly regulated by various kinases and phosphatases. The enzymatic activity of Ciona intestinalis voltage sensor-containing phosphatase (Ci-VSP), recently identified as a member of the PTEN (phosphatase and tensin homolog deleted on chromosome 10) family of phosphatidylinositol phosphatases, is regulated by its own voltage-sensor domain in a voltagedependent manner. However, a detailed mechanism of Ci-VSP regulation and its substrate specificity remain unknown. Here we determined the in vitro substrate specificity of Ci-VSP by measuring the phosphoinositide phosphatase activity of the Ci-VSP cytoplasmic phosphatase domain. Despite the high degree of identity shared between the active sites of PTEN and Ci-VSP, Ci-VSP dephosphorylates not only the PTEN substrate, phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], but also, unlike PTEN, phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Enzymatic action on PI(4,5)P2 removes the phosphate at position 5 of the inositol ring, resulting in the production of phosphatidylinositol 4-phosphate [PI(4)P]. The active site Cys-X5-Arg (CX5R) sequence of Ci-VSP differs with that of PTEN only at amino acid 365 where a glycine residue in Ci-VSP is replaced by an alanine in PTEN. Ci-VSP with a G365A mutation no longer dephosphorylates PI(4,5)P2 and is not capable of inducing depolarization-dependent rundown of a PI(4,5)P2-dependent potassium channel. These results indicate that Ci-VSP is a PI(3,4,5)P3 ⁄ PI(4,5)P2 phosphatase that uniquely functions in the voltage-dependent regulation of ion channels through regulation of PI(4,5)P2 levels.
Phosphatidylinositol lipids play diverse physiological roles, and their concentrations are tightly regulated by various kinases and phosphatases. The enzymatic activity of Ciona intestinalis voltage sensor-containing phosphatase (Ci-VSP), recently identified as a member of the PTEN (phosphatase and tensin homolog deleted on chromosome 10) family of phosphatidylinositol phosphatases, is regulated by its own voltage-sensor domain in a voltagedependent manner. However, a detailed mechanism of Ci-VSP regulation and its substrate specificity remain unknown. Here we determined the in vitro substrate specificity of Ci-VSP by measuring the phosphoinositide phosphatase activity of the Ci-VSP cytoplasmic phosphatase domain. Despite the high degree of identity shared between the active sites of PTEN and Ci-VSP, Ci-VSP dephosphorylates not only the PTEN substrate, phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], but also, unlike PTEN, phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Enzymatic action on PI(4,5)P2 removes the phosphate at position 5 of the inositol ring, resulting in the production of phosphatidylinositol 4-phosphate [PI(4)P]. The active site Cys-X5-Arg (CX5R) sequence of Ci-VSP differs with that of PTEN only at amino acid 365 where a glycine residue in Ci-VSP is replaced by an alanine in PTEN. Ci-VSP with a G365A mutation no longer dephosphorylates PI(4,5)P2 and is not capable of inducing depolarization-dependent rundown of a PI(4,5)P2-dependent potassium channel. These results indicate that Ci-VSP is a PI(3,4,5)P3 ⁄ PI(4,5)P2 phosphatase that uniquely functions in the voltage-dependent regulation of ion channels through regulation of PI(4,5)P2 levels. Published in Proceedings of the National Academy of Sciences (online) on June 2.
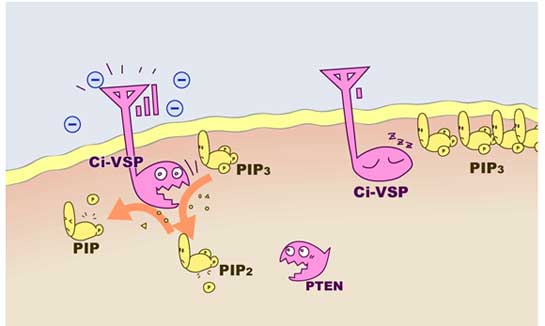
Ci-VSP, voltage-sensing phosphatase, has a similar catalytic site with PTEN, but it can remove a phosphate not only from PIP3, but also from PIP2.
 (C)KOIDA, Kowa
(C)KOIDA, Kowa