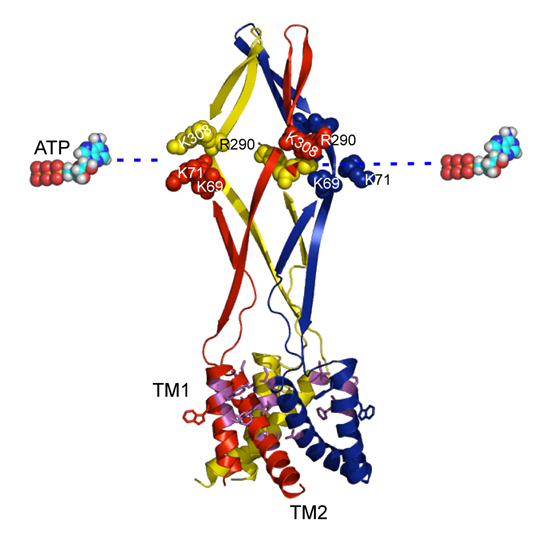
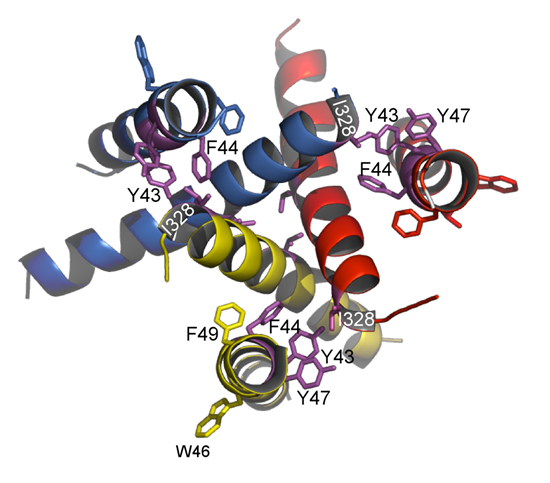
The extracellular ATP-gated cation channel P2X2 is known to show voltage- dependent gating in spite of the absence of a canonical voltage sensor domain. We previously observed that the hyperpolarization-evoked activation of P2X2 at the steady state in the presence of ATP varied depending on [ATP]. With increasing [ATP], the conductance–voltage (G–V) relationship shifted to more depolarized potentials and the activation kinetics were accelerated. Using a three-state model consisting of an ATP binding step and a rate limiting gating step, we successfully reproduced the voltage-dependent gating including its [ATP] dependence. In this study, in order to identify the structural basis of voltage and ATP dependence, we analysed various mutants in the Xenopus oocyte expression system under two-electrode voltage clamp. In the ATP binding region mutant K308R, the G–V relationship was shifted towards more hyperpolarized potentials, there was no clear [ATP] dependence, and activation was faster than in wild-type (WT). These results could be simulated by assuming an increase in the off rate of the gating step, in addition to changes in the ATP binding step. With F44C mutation in the 1st transmembrane (TM) region (TM1) or T339S in TM2, activation in low [ATP] was slow and the channel was constitutively active at all membrane potentials in high [ATP]. These results could be simulated by reducing the off rate of the gating step. Phenotypes of the double mutants, K308R/F44C and K308R/T339S, were similar to WT, suggesting that TM and ATP binding region mutants offset the effect of each other. Analysis of the effects on WT of two other agonists, ADP and AP4A, revealed that the electrostatic charge is not the sole critical factor. Taking these results together with the recently reported crystal structure, it was suggested that upon binding of ATP, the occupied binding site indirectly interacts with the extracellular end of the TM regions to trigger conformational changes for gating in a voltage-dependent manner.
Batu Keceli and Yoshihiro Kubo
Functional and structural identification of amino acid residues of the P2X2 receptor channel critical for the voltage- and [ATP]-dependent gating.
Journal of Physiology 587: 5801-5818 (2009)
Based on the crystal structure of the zebra fish P2X4 by Kawate et al. (2009), we performed a homology modeling of the rat P2X2 and mapped critical amino acid residues on the structure. (A) A side view. (B) A view of the external end of the