The gamma-aminobutyric acid type B receptor (GABABR), one of the family C G-protein-coupled receptor members, exists as a heterodimer comprised of subunits GB1 and GB2. To clarify the ligand-induced activation mechanism of the GABABR, each subunit was fused with either Cerulean or enhanced yellow fluorescent protein at its intracellular loop, and fluorescence resonance energy transfer (FRET) changes upon agonist application were monitored. As a result, FRET decreases were observed between GB1a loop 2 (i2) and GB2 loop 2 (i2) and between GB1a loop 2 (i2) and GB2 loop 1 (i1), suggesting the dissociation of intracellular domains during the receptor activation. Both intersubunit FRET pairs were expected to faithfully capture the activation of original receptor as their pharmacological properties were highly similar to that of wild-type receptor. However, the intrasubunit data suggest that the receptor activation does not involve major structural changes within the transmembrane domain of each subunit. By combining the results obtained from two different levels, it was concluded that the GABABR activation by agonist is associated with an asymmetrical intersubunit rearrangement of GB1a and GB2 on the membrane. This type of activation mode, an intersubunit rearrangement without apparent intrahelical structural changes, appears commonly shared by the GABABR and the metabotropic glutamate receptor type 1, another family C G-protein-coupled receptor previously studied by our group. Nevertheless, the directions of intracellular domain movements and its asymmetry observed here highlight the qualitative difference between the two receptors.
Shinichi Matsushita, Hiroyasu Nakata, Yoshihiro Kubo, and Michihiro Tateyama Ligand-induced rearrangements of the GABAB receptor revealed by fluorescence resonance energy transfer
The Journal of Biological Chemistry, 285(14): 10291-10299 (2010)
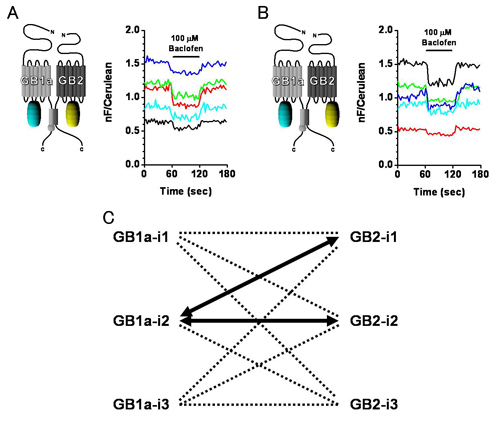
Both GB1a-i2 & GB2-i2 (A) and GB1a-i2 & GB2-i1 (B) pairs exhibit FRET decreases upon agonist application. C, Combinations of intracellular loops from which FRET changes were seen (thick arrows).
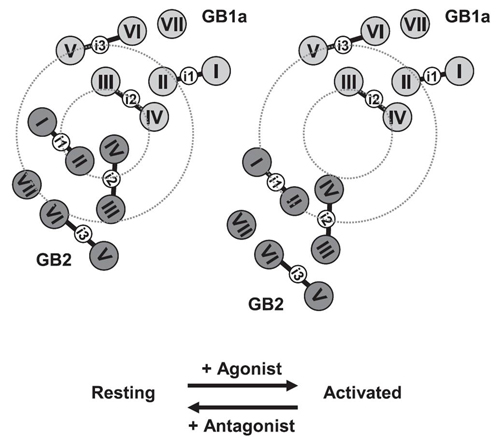
Activation model of GABABR based on Figure 1.p>