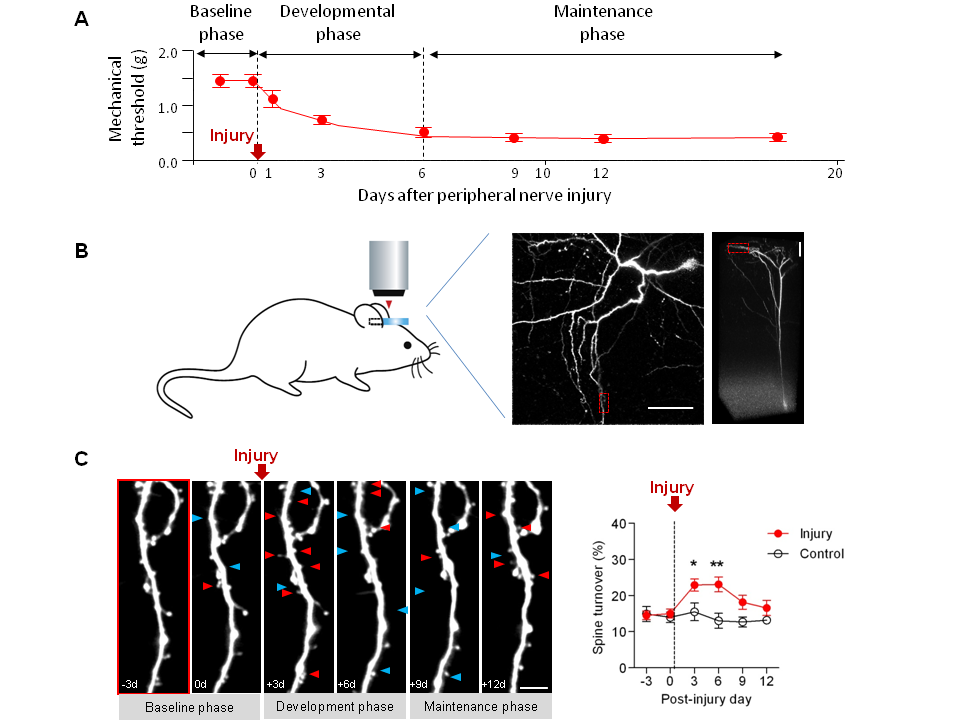
Peripheral nerve injury often leads to chronic neuropathic pain, which is characterized by tactile allodynia (a painful response to usually innocuous mechanical stimuli) and involves structural and functional plastic changes in the primary somatosensory (S1) cortex. However, remodeling of cortical connections following injury has been believed to take months or years, which is not temporally correlated with the rapid development of allodynia and S1 hyperexcitability (days or weeks).
In this study, we used long-term in vivo two-photon imaging to longitudinally trace the dynamics of postsynaptic dendritic spines in the S1 cortex of living adult mice with neuropathic pain induced by the partial sciatic nerve ligation injury, a well-characterized animal models. Spine turnover (formation and elimination) in the S1 area corresponding to the injured paw markedly increased during early development phase of neuropathic pain and was restored in later maintenance phase of neuropathic pain, which was prevented by immediate local blockade of the injured nerve throughout development phase. New spines that generated just before nerve injury showed volume decrease after injury, whereas more new spines that formed in development phase became persistent and substantially increased their volume during maintenance phase. Further, pre-existing stable spines survived less following injury than controls and such lost persistent spines were smaller in size than the survived ones that displayed long-term potentiation-like enlargement over weeks. These results suggest that peripheral nerve injury induce rapid and selective remodeling of cortical synapses, which is associated with neuropathic pain development, probably underlying, at least partially, long-lasting sensory changes in neuropathic subjects. These results not only show the structural and temporal correlates of neuropathic pain at individual synapse levels in the cortex, but also provide a potential therapeutic target in intractable chronic pain.
The Journal of Neuroscience, April 6, 2011 • 31(14):5477–5482
Sun Kwang Kim and Junichi Nabekura