Individual cells in living organisms may vary their shape, begin their proliferation or migration, or, in some cases, lead themselves to cell death (apoptosis) in response to external stimuli. During execution of these processes, the volume of the cell is neatly regulated. Cell volume regulation is attained by regulating the net influx or outflux of water and solutes across the cell membrane. The volume-sensitive outwardly rectifying (VSOR) anion channel is a major regulator of anion transport during the regulation and expressed in almost all types of animal cells. We previously demonstrated that the VSOR channels in the major glial cells, astrocytes, are activated by an inflammatory chemical mediator, bradykinin, and that the channels may also serve as a pathway for the release of an excitatory amino acid, glutamate, thereby participating in signal transmission to adjacent neurons (Liu et al. (2009) J Physiol 587(10):2197-2209). Recently, we found that this VSOR channel activation is regulated in the immediate vicinity of individual Ca2+ channel proteins in the cells.
Through binding to Gq protein-coupled bradykinin B2 receptors, bradykinin induces the opening of IP3-receptor Ca2+ channels on intracellular Ca2+ stores and then store- or receptor-operated Ca2+ channels in the plasma membrane. In the very high Ca2+ concentration regions created within ~20 nm of open Ca2+ channels, called ‘Ca2+ nanodomains’, Ca2+-dependent protein kinases (PKC and are found to be activated, and these kinases are involved in VSOR channel activation through inducing generation of reactive oxygen species (ROS) by NADPH oxidases (NOX; figure 1).
This Ca2+ nanodomain-mediated mechanism of VSOR channel activation ensures generation of activating signals around individual open Ca2+ channels even when only a minute amount of bradykinin acts on a part of the cell and a small number of Ca2+ channels are opened. Furthermore, because the activation is mediated by the enzyme cascade, VSOR channel activity is maintained for a while even after most of the Ca2+ channels have closed. Thus the mechanism is considered to play important roles in local control of cell volume regulation, i.e. cell shape changes, and of intercellular communications on a long time scale.
Akita T and Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589(16):3909-3927.
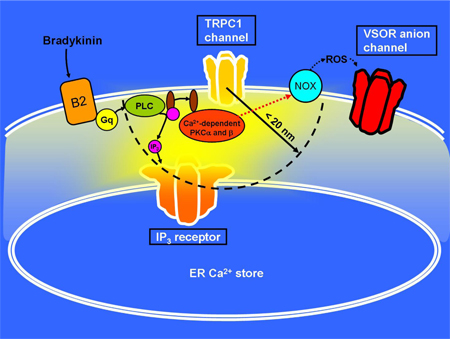
Cartoon of Ca2+ nanodomain-mediated activation of VSOR anion channels in mouse astrocytes
When bradykinin binds to the B2 receptors in the cell membrane, the Gq proteins coupled to the receptors activate phospholipase C (PLC), which produces inositol 1,4,5-trisphosphate (IP3) in the cytoplasm. Then IP3-receptor Ca2+ channels on intracellular endoplasmic reticulum (ER) Ca2+ stores are opened, and this further induces the opening of several types of store- or receptor-operated Ca2+ channel in the cell membrane. Among these Ca2+ channels, especially IP3 receptors and TRPC1 channels are found to be strongly involved in VSOR channel activation. Within 20 nm (1 nm = 10–6 mm) of these channels, Ca2+-dependent PKC and are activated, and these PKCs subsequently activate NOX which generates ROS for VSOR channel activation.