KCNQ1 channels are voltage-gated potassium channels that are widely expressed in various non-neuronal tissues, such as the heart, pancreas, and intestine. KCNE proteins are known as the auxiliary subunits for KCNQ1 channels. The effects and functions of the different KCNE proteins on KCNQ1 modulation are various; the KCNQ1–KCNE1 ion channel complex produces a slowly activating potassium channel that is crucial for heartbeat regulation, while the KCNE3 protein makes KCNQ1 channels constitutively active, which is important for K+ and Cl− transport in the intestine. The mechanisms by which KCNE proteins modulate KCNQ1 channels have long been studied and discussed; however, it is not well understood how different KCNE proteins exert considerably different effects on KCNQ1 channels. Here, we approached this point by taking advantage of the recently isolated Ci-KCNQ1, a KCNQ1 homologue from marine invertebrate Ciona intestinalis. We found that Ci-KCNQ1 alone could be expressed in Xenopus laevis oocytes and produced a voltage-dependent potassium current, but that Ci-KCNQ1 was not properly modulated by KCNE1 and totally unaffected by coexpression of KCNE3. By making chimeras of Ci-KCNQ1 and human KCNQ1, we determined several amino acid residues located in the pore region of human KCNQ1 involved in KCNE1 modulation. Interestingly, though, these amino acid residues of the pore region are not important for KCNE3 modulation, and we subsequently found that the S1 segment plays an important role in making KCNQ1 channels constitutively active by KCNE3. Our findings indicate that different KCNE proteins use different domains of KCNQ1 channels, and that may explain why different KCNE proteins give quite different outcomes by forming a complex with KCNQ1 channels.
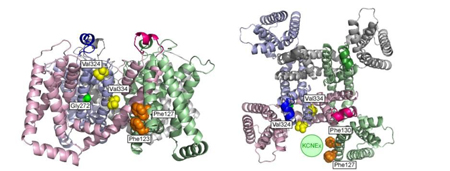
Figure Legend:Mapping of the interaction sites for KCNE1 and KCNE3 on KCNQ1 channel
Side view (left) and top view (right) of tetrameric KCNQ1 channel are shown. Green circle is a hypothetical location of KCNE protein. KCNE1 may interact with Gly272 (green), Val324 and Val 334 (yellow); all are located on the pore domain. KCNE3 may interact with Phe123, Phe127 and Phe130 on the S1 segment (Phe130 of the left figure and Phe123 of the right figure are behind the other phenylalanine residues).
KCNQ1 subdomains involved in KCNE modulation revealed by an invertebrate KCNQ1 ortholog.
Koichi Nakajo, Atsuo Nishino, Yasushi Okamura and Yoshihiro Kubo.
Journal of General Physiology 138: 521-535, 2011.