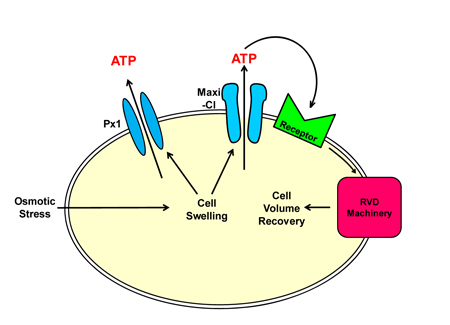
ATP is not only a biochemical energy source in cells but also serves as an extracellular signaling molecule. Under various kinds of stresses, including osmotic stress, cells release ATP into the extracellular space. Released ATP then binds to its receptor, located on the outer surface of the cell membrane, and thereby exerts the signaling action on the same cell and/or the neighboring cells in an autocrine and/or paracrine fashion, respectively. ATP can be released through the exocytosis mechanism where ATP-containing vesicles fuse with the cell membrane and pour ATP in the extracellular side. Also, a major fraction of cytoplasmic ATP is released through the pore of the channel proteins embedded in the cell membrane. Connexin 43 (Cx43), which ordinarily forms a gap junction between two adjacent cells together with another Cx43 on the partner cell, has been reported to form a hemichannel alone on the cell and serve as an ATP-releasing pathway. Similarly pannexin 1 (Px1) forms a hemichannel and acts as another ATP gateway. In addition, under some pathophysiological conditions, the maxi-anion channel (Maxi-Cl), which has a large pore and exhibits a large single-channel conductance, has recently been reported as a vital ATP-conducting channel in various types of cells. In spite of the significant role, its molecular entity is still unidentified. Because of a similar ATP-releasing function, Px1 or Cx43 hemichannel has been attracted much attention as a possible candidate of the Maxi-Cl molecule.
The present study conducted mainly by Md. Rafiqul Islam, a Foreign Researcher of NIPS who is from Bangladesh, using L929 fibrosarcoma cells (so-called L cells), reported following new findings. First, ATP is released neither via Px2 nor by the exocytosis mechanism and little via Cx43, but Maxi-Cl and Px1 are significantly involved as ATP-releasing conduits in parallel. Second, ATP released via Maxi-Cl, but not Px1, plays an essential role in the cell volume regulation after osmotic swelling, called regulatory volume decrease (RVD). Thus, it is conceivable that Maxi-Cl is located in a closer vicinity to the cellular RVD machinery than Px1 (see figure below). Third, the molecular entity of Maxi-Cl is distinct from Px1, Px2 and Cx43.
The same issue of the journal, published by the American Physiological Society, carried an article of Editorial Focus in response to the paper by Islam et al. and highlighted the notable achievement of this work with putting an emphasis on the molecular identification of the desired maxi-anion channel: Dubyak GR (2012) Function without form: An ongoing search for maxi-anion channel proteins. Am J Physiol Cell Physiol 303: C913-C915 (doi: 10.1152/ajpcell.00285.2012)
Islam MR, Uramoto H, Okada T, Sabirov RZ & Okada Y (2012) Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol 303: C924-C935 (doi: 10.1152/ajpcell.00459.2011)