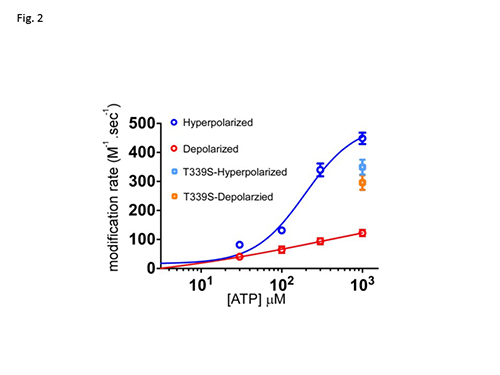
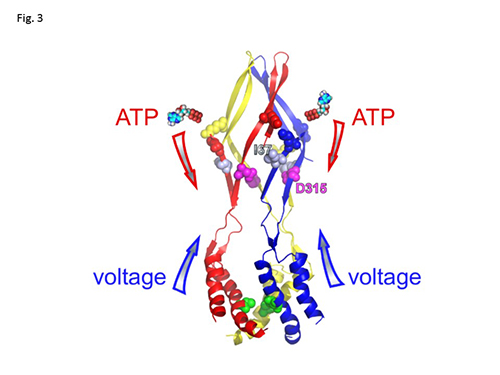
P2X2 is an extracellular ATP gated cation channel, which has a voltage-dependent gating property even though it lacks a canonical voltage sensor. P2X2 is a trimer in which each subunit has two transmembrane helices and a large extracellular domain. The three inter-subunit ATP binding sites are linked to the pore forming transmembrane (TM) domains by β-strands. We analyzed structural rearrangements of the linker strands between the ATP binding site and TM domains upon ligand binding and voltage change, electrophysiologically in Xenopus oocytes, using mutants carrying engineered thiol-modifiable cysteine residues. (1) We demonstrated that the double mutant D315C&I67C (at β-14 and β-1, respectively) shows a 2-4 fold increase in current amplitude after treatment with a reducing reagent, dithiothreitol (DTT). Application of the thiol-reactive metal Cd2+ induced current decline due to bond formation between D315C and I67C. This effect was not observed in WT or in single point mutants. (2) Cd2+ induced current decline was analyzed in hyperpolarized and depolarized conditions with different pulse protocols, and also in the presence and absence of ATP. (3) Current decline by Cd2+ could be clearly observed in the presence of ATP. However, it was not clear in the absence of ATP, showing a state dependent modification. (4) In the presence of ATP, Cd2+ modification was significantly faster in hyperpolarized than in depolarized conditions (Fig.1), showing voltage-dependent structural rearrangements of the linker strands. (5) The bridging by Cd2+ between 315 and 67 was shown to be not intra- but inter- subunit, by experiments using tandem trimeric constructs (TTC) with controlled number and position of mutations in the trimer. (6) Finally, we performed similar analyses of a pore mutant T339S, which makes the channel activation voltage insensitive. Cd2+ modification rates of T339S were similar in hyperpolarized and depolarized conditions (Fig. 2). Taking these results together, we demonstrated that structural rearrangements of the linker region of the P2X2 receptor channel are induced not only by ligand binding but also by membrane potential change (Fig. 3).
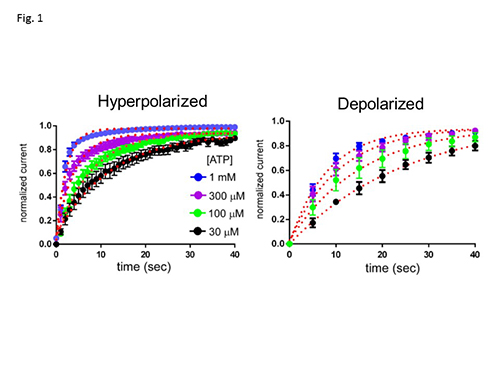 The modification speed of Cys mutant (D315C&I67C) by Cd2+ changes depending on the membrane potential and ATP concentration.
The modification speed of Cys mutant (D315C&I67C) by Cd2+ changes depending on the membrane potential and ATP concentration.
Batu Keceli and Yoshihiro Kubo
J Physiol September 2014; doi:10.1113/jphysiol.2014.278507