Researchers in Japan elucidated a novel molecular mechanism in placental calcium transport. Hypomorphic TRPV6 gene variants interfere with maternal-fetal calcium transport, and cause transient neonatal hyperparathyroidism.
Abstract:
Scientist from Japan’s National Institute for Physiological Sciences and their collaborators have identified a novel molecular mechanism in placental (maternal-fetal) calcium transport, which plays a pivotal role in fetal bone mineralization. They have found that hypomorphic TRPV6 gene variants interfere with placental calcium transport, and thus impair fetal bone mineralization, which clinical consequence is known as transient neonatal hyperparathyroidism
Okazaki-Japan –
Calcium is a critical ion in our body. It serves as an intracellular signaling factor, cofactor for numerous enzymes and proteins, and a major bone mineral. Fetuses need calcium for their proper development of the nervous system, muscles, and other organs. In parallel, fetuses need plenty of calcium for fetal bone development during late pregnancy. It is postulated that fetuses require 300 mg/day of calcium. The large amount of calcium is supplied by maternal-fetal placental calcium transport (one of the super-powers of expecting mothers). However, the molecular mechanism of the super-power has not been fully understood.
Transient neonatal hyperparathyroidism is a rare bone-endocrine disorder, which manifests with severely decreased bone mineralization, associated with bone deformities, fractures, and even respiratory failure due to abnormal ribs. The disorder was mainly attributed to maternal hypocalcemia. However, a number of affected babies born to normocalcemic mothers have been reported. The etiology of such cases has remained determined. In this study, the researchers have found hypomorphic TRPV6 gene variants in a neonate with transient neonatal hyperparathyroidism by using whole exome sequencing. Following molecular analyses for additional patients have elucidated that TRPV6 mutations are a common cause of the disorder. TRPV6 gene encodes a calcium-selective ion channel that expresses calcium transporting-epithelium such as the small intestine and placenta. Based on further basic investigations, the researchers also clarified pathogenic mechanisms affecting TRPV6 function, including the plasma membrane expression, protein stability, and intracellular calcium-dependent inactivation resulting in the Ca2+ overload.
These results indicate that TRPV6 is important for the maternal-fetal calcium transport and fetal bone mineralization. This study leads not only to the better diagnosis of transient neonatal hyperparathyroidism but also new treatment, e.g., development of TRPV6-targeted compounds. In addition, this study implies that babies who are diagnosed as having neonatal rickets (vitamin D deficiency) may have the impaired calcium transport.
Brief summary:
Contribution to society:
This discovery facilitates the diagnosis and medical management for transient neonatal hyperparathyroidism. Moreover, we can expect development of targeted medication for the disorder.
TRPV6 variants interfere with the maternal-fetal calcium transport through the placenta causing neonatal transient hyperparathyroidism.
Yoshiro Suzuki , David Chitayat, Hirotake Sawada, Matthew A. Deardroff, Heather M. McLaughlin, Amber Begtrup, Kathryn Millar, Jennifer Harrington, Karen Chong, Maian Roifman, Katheryn Grand, Makoto Tominaga, Fumio Takada, Shirley Shuster, Megumi Obara, Hiroshi Mutoh, Reiko Kushima, Gen Nishimura.
Am. J. Hum. Genet.
11:00 Eastern Time on 31st May (0:00 Japanese Time on 1st June)
Keywords:
maternal-fetal calcium transport, TRPV6, neonatal transient hyperparathyroidism, PTH, placenta, fetal bone mineralization
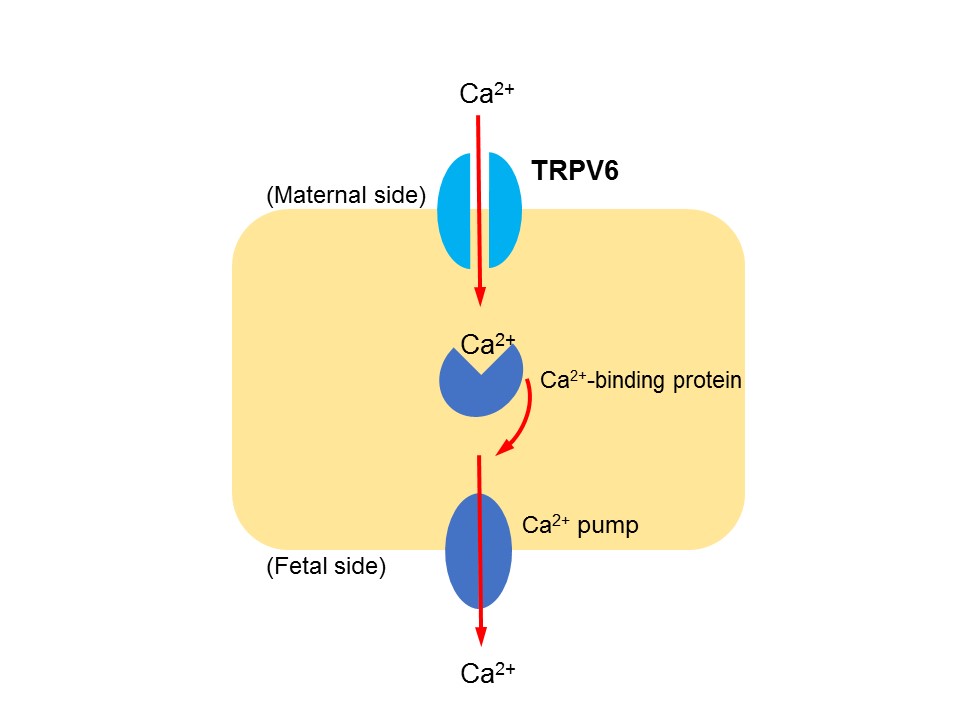
It has been well known that maternal-fetal placental calcium (Ca2+) transport during late pregnancy is important for normal fetal bone mineralization. However, the molecular mechanism of the placental calcium transport has not been fully understood. In this study, we found that an apical Ca2+ through TRPV6 plays an important role in maternal-fetal Ca2+ transport.



National Institute for Physiological Sciences (NIPS)
University of Miyqazaki
Saitama Medical University