Activation mechanisms of GIRK channel by an antiparasitic drug, ivermectin.
Ivermectin (IVM), a worldwide used antiparasitic drug that rescues humans and pets from parasite infections, was discovered by Dr. Omura and Dr. Campbell to whom the 2015 Nobel Prize in Physiology or Medicine was awarded. IVM kills parasites by activating glutamate-gated Cl- (GluCl) channel by binding to its transmembrane domains (TMs) (Figure, left panel). It is known that IVM also binds to the TMs of several ligand-gated channels, such as Cys-loop receptors and ATP receptor channel P2X.
In this study, we found that the G-protein-gated inwardly rectifying K+ (GIRK) channel, especially GIRK2, is activated by IVM directly. The activation is independent of Gβγ, but dependent on phosphatidylinositol-4,5-biphosphate (PIP2). In primary cultured neurons of rat hippocampus, we also observed that IVM activates native GIRK current. We identified, by mutagenesis analyses, a critical amino acid residue of GIRK2 for activation by IVM. It is Ile82 located in the slide helix between the TM1 and the N-terminal cytoplasmic tail domain (CTD) (Figure, right panel).
The results demonstrate that the TM–CTD interface in GIRK channel, rather than the TMs, governs IVM-mediated activation and provide us with novel insights concerning about the mode of action of IVM on ion channels.
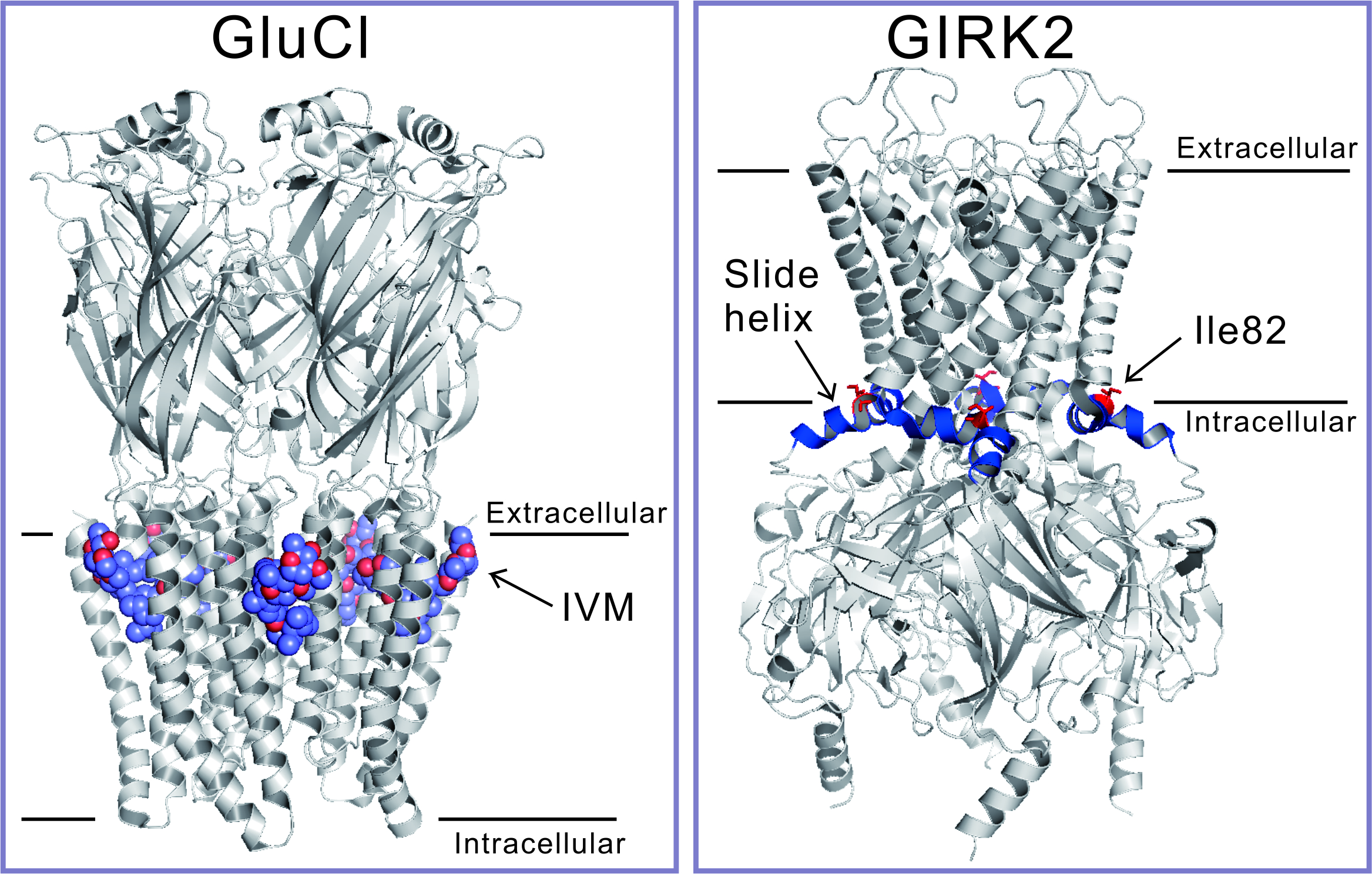
Figure:IVM-binding site in GluCl channel (left panel) and the critical amino acid residue for IVM effect in GIRK2 channel (right panel)
Collaborative Researcher
Yuko Fukata (Division of Membrane Physiology, NIPS)
Motonari Uesugi (WPI-iCeMS, Kyoto University)

