The unique structures of sodium ion transporting V-ATPase revealed by Zernike phase-contrast cryo-electron microscopy
All living things keep the solution composition ratio constant inside the cells, especially the concentration of ions is critically controlled. When there is irritation from the outside, this solution composition ratio is temporarily disturbed, and it can be felt as stimulation. For that purpose, the cell uses an energy of ATP to control the concentration of ions in the cell. One of the proteins that play this role is V-ATPase.
The Eh V-ATPase used in this study is a V-ATPase present in the cell membrane of Enterococcus hirae, and selectively excretes sodium ions to outside of the cell. The entire structure of Eh V-ATPase was unknown, where the partial structures of Eh V-ATPase was only revealed by X-ray crystallographic structural analysis. This was because the particle image of the solubilized Eh V-ATPase was not able to be visualized by conventional cryo-electron microscopy which was the only way to see the entire structure of the complex.
In this study, we succeeded in visualizing the particle image of Eh V-ATPase solubilized from cell membrane for the first time, using Zernike phase contrast cryo-electron microscopy developed at NIPS. Then, the entire structure of Eh V-ATPase was reconstructed by single particle analysis by using the particle images. V-ATPases generally show machine-like structure in which a rotary domain using ATP hydrolysis energy called V1 and another ion-pump rotary motor called Vo are connected by a single rotor. Here, we revealed the unique features of Eh V-ATPase, where the enzyme has V1 and Vo domains fixed with two stalks, and the central rotor extending from V1 is connected to the ring of Vo rotor with the off-axis manner. In addition, the structure artificially fixed the central rotor by binding the antibody revealed the second stable structure among three consecutive structural states of this enzyme.
Based on these results, we could clarify the structural basis of energy transduction mechanism for sodium ion transport of Eh V-ATPase. By further structural research, it is expected that it will be able to provide important information in the treatment of diseases related to V-ATPase and development of therapeutic drugs in the future.
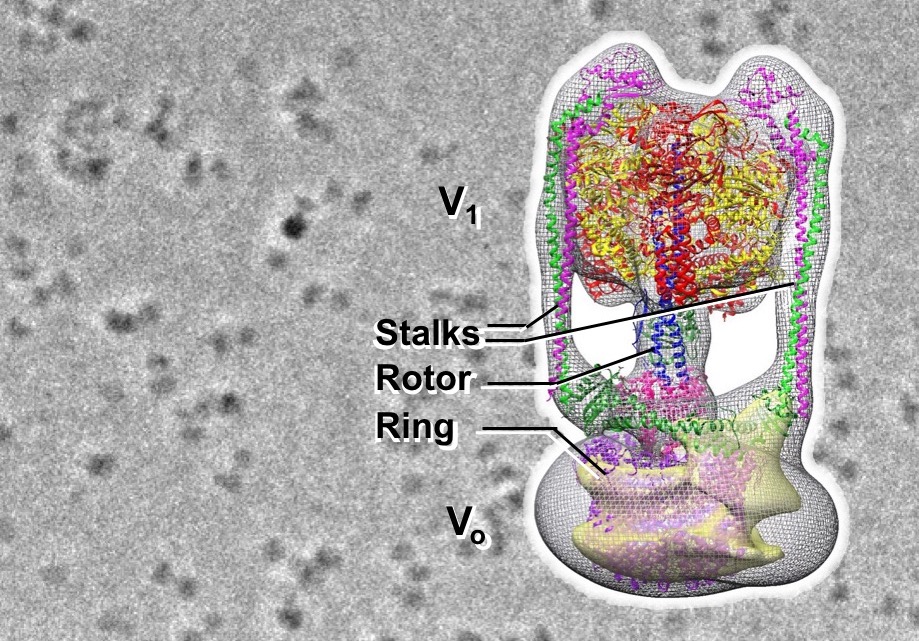
Figure: Image of Eh V-ATPase by Zernike phase contrast cryo-electron microscopy (background) and the 3D structure
Collaborative Researchers
Ryota Iino (IMS)
Fabiana Lica Imai, Takeshi Murata (Chiba Univ.)
Hiroshi Ueno (Univ Tokyo)
Junichi Takagi (Osaka Univ)
Jun Tsunoda (NIPS)
Grants
KAKENHI, Advanced Bioimaging Support (ABiS), JST-ERATO Momose Quantum-beam Phase Imaging Project, Collaborative Study Program of the National Institute for Physiological Sciences,

