An approach to the mechanism of assembly and disassembly of the human proteasome complex
Proteasomes are protein complexes that degrade unneeded or damaged proteins by proteolysis in cells. It exhibits a cage-like structure commonly shown from bacteria to humans, in which unnecessary proteins are trapped and degraded. In the human proteasome, an α and β rings consisted of seven slightly different proteins are linked together to form a cage with α-β-β-α arrangement. These seven proteins in each ring are sequentially numbered as α1-7 and β1-7, and thus the human proteasome consists of a total of 14 different proteins. Here, the question is asked, “Why can 14 different proteins be ordered faithfully and form such a cage without mistakes?” In this study, the collaborative research team led by Professor Koichi Kato in ExCELLS approached to the formation mechanism of the proteasome cage by using molecular biology and biophysical methods.
From the team's previous research, it was found that seven of the α7 proteins form a ring structure, and the two further overlap to form a double ring structure. Furthermore, it was also known that α6 disrupts the double ring into a single ring. In this study, it was found that α4 also disrupted the α7 double ring to the single ring. Furthermore, α7 mutationally modified the amino acids located inside the double ring to other amino acids successfully showed a monomerized α7 (hereinafter α7*). Interestingly, the addition of α6 converted the monomeric proteins to a double ring consisting of 14 proteins. The same effect was seen with additions of α4. In collaboration with NIPS, it was shown by electron microscopy that the double ring structure of α7* was formed by the addition of α4. In summary, our data indicated that the proteasomal subunits can be combinatorically assembled into a variety of protein complexes.
Our results are expected to greatly contribute to the design of molecular machines using building blocks of proteins, as well as to provide insights into the molecular mechanisms of dissociation and association of human proteasome and their functions, which are acquired during evolution.
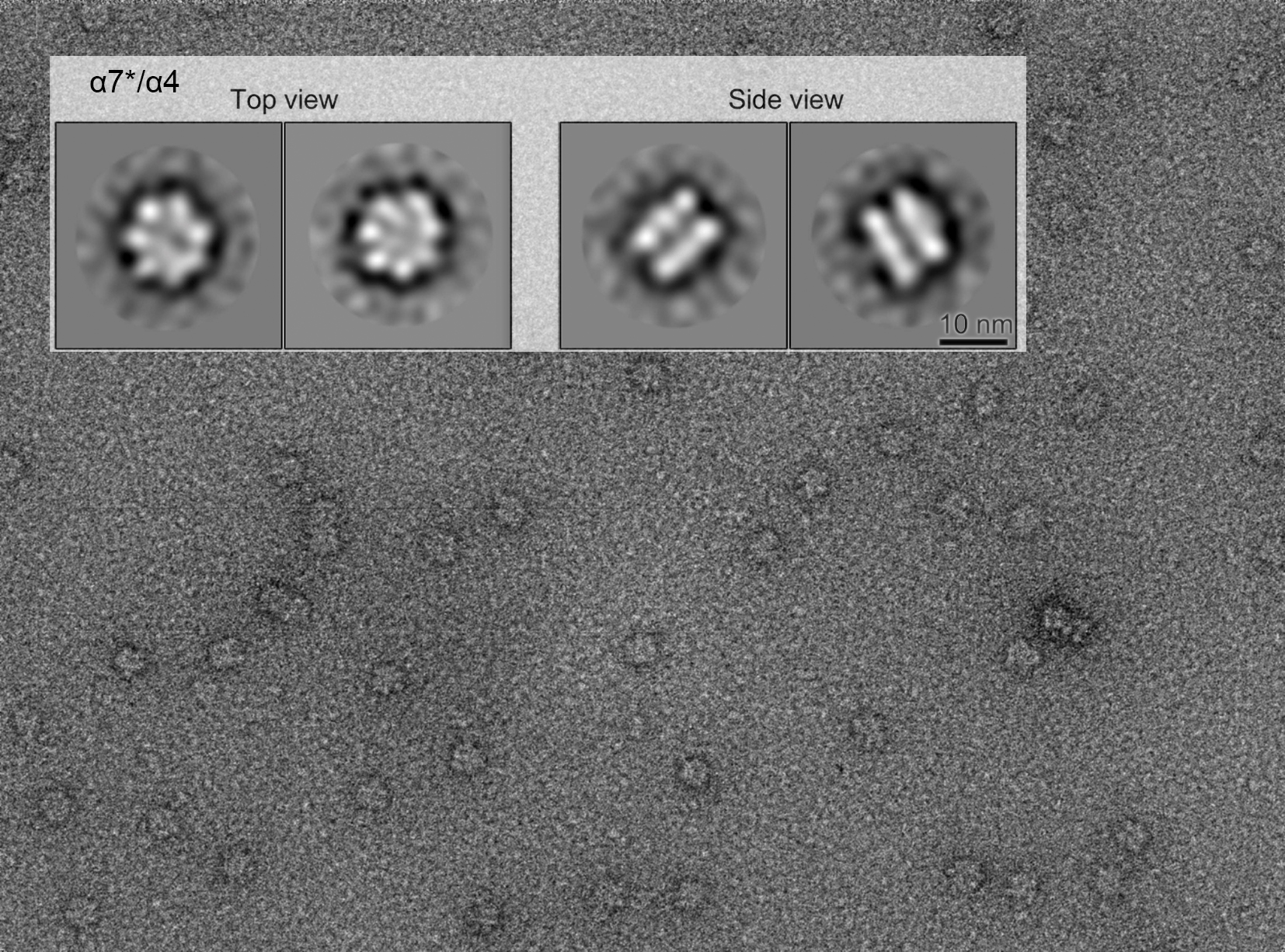 Figure Genetically modified monomerized human proteasome α7 (α7*) formed a double ring by the addition of α4.
Figure Genetically modified monomerized human proteasome α7 (α7*) formed a double ring by the addition of α4.
Collaborative Researcher
Tadashi Satoh, Hirokazu Yagi (Nagoya City University)
Saeko Yanaka, Koichi Kato (ExCELLS))
Funding
KAKENHI, ExCELLS, SOKENDAI
Release Source
Title: Mutational and Combinatorial Control of Self-Assembling and Disassembling of Human ProteasomeαSubunits
Authors: Taichiro Sekiguchi, Tadashi Satoh, Eiji Kurimoto, Chihong Song, Toshiya Kozai, Hiroki Watanabe, Kentaro Ishii, Hirokazu Yagi, Saeko Yanaka, Susumu Uchiyama, Takayuki Uchihashi, Kazuyoshi Murata and Koichi Kato
Journal: International Journal of Molecular Sciences
Issue: 20(9), 2308
Date: 2019, May 9 Published
URL: https://doi.org/10.3390/ijms20092308
DOI: 10.3390/ijms20092308.

