Why Helicobacter pylori phage is not digested in the stomach?
-Expectations for phage therapy-
2021.10.26
Helicobacter pylori is a bacterium that causes gastric ulcers and gastric cancer. H. pylori phage is a virus that hosts H. pylori, which has the ability to selectively kill H. pylori causing gastric ulcer and gastric cancer. Therefore, eradication of H. pylori by administration of H. pylori phage is attracting attention as a new therapeutic method called “phage therapy”, different from the mechanism of action for conventional antimicrobial agents. However, in order to realize this therapy, it is necessary to collect detailed knowledge about the morphology of this phage itself and the mechanisms of infection and proliferation.
In this joint research, the molecular structure of the head (capsid) that stores the gene for the H. pyroli phage named KHP30 was clarified at a resolution of 0.27 nm using single particle cryo-electron microscopy (cryo-EM)* (Fig. 1). The major capsid proteins (MCPs) form strong hexamers (navy blue, purple in Fig. 1) or pentamers (cyan in Fig. 1) in which a special loop (P-loop) provided from the MCP functions as a wedge between adjacent molecules, covering the surface of the head. The gaps between hexamers and pentamers were double-covered by the celemt proteins of the trimer (green in Fig. 1).
This result structurally clarified the mechanism by which H. pylori phages are not destroyed even in a strongly acidic stomach. In addition, by applying this, it is expected to pave the way for the practical application of this phage therapy without worrying about drug resistance due to overdose of antibacterial drugs.
* single particle cryo-EM:
Micrographs of rapid-frozen samples were directly collected with a transmission electron microscope. The individual images of the sample particles are then averaged and reconstructed as 3D in a computer.
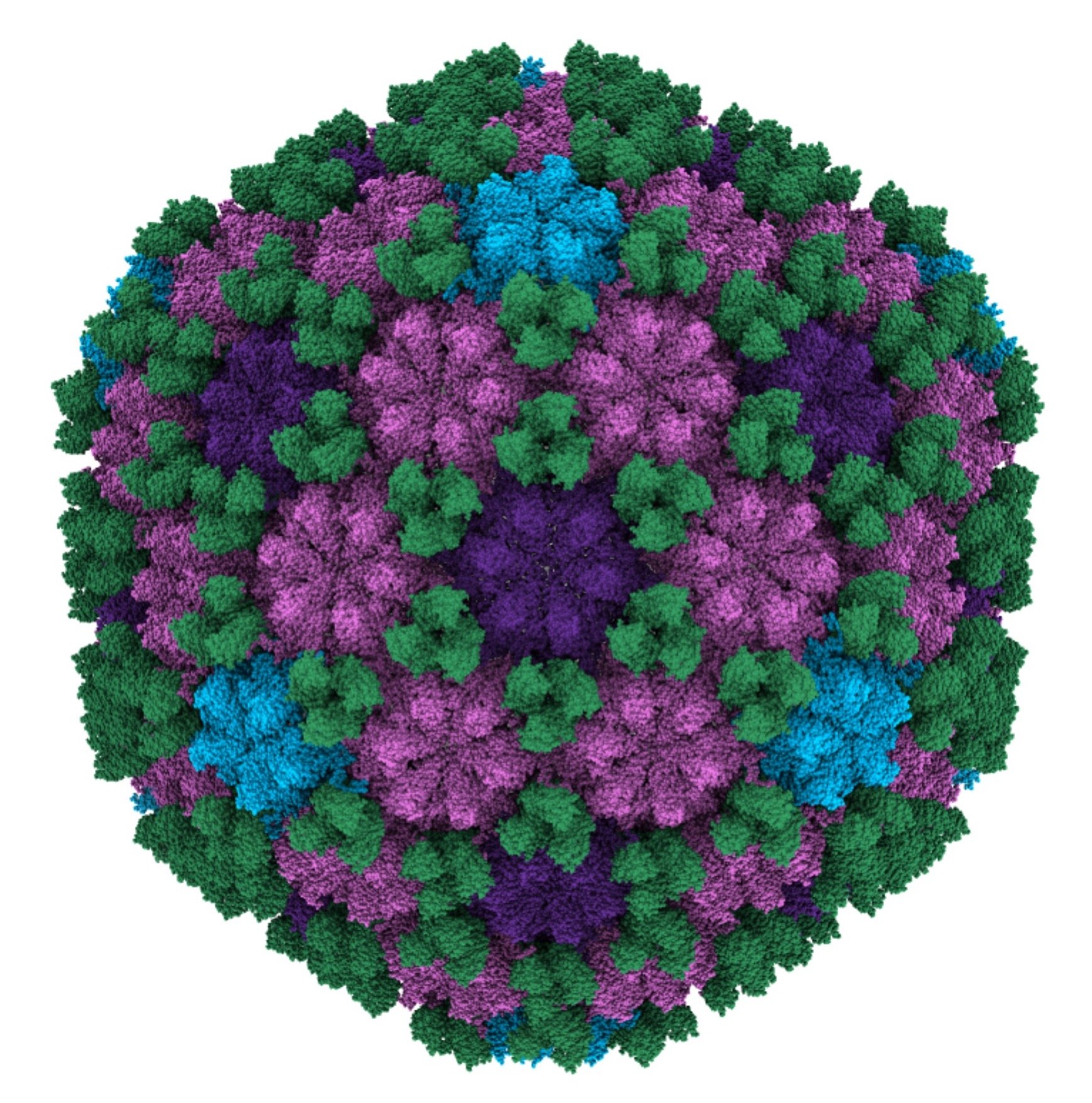
Figure 1. A cryo-EM map of the H. pylori phage KHP30 head
Collaborative Researchers
Naoyuki, Miyazaki (Univ. Tsukuba)
Junpei Uchiyama (Azabu Univ., Okayama Univ. at present)
Kazuyoshi Murata (NIPS/ExCELLS/ SOKENDAI)
Junpei Uchiyama (Azabu Univ., Okayama Univ. at present)
Kazuyoshi Murata (NIPS/ExCELLS/ SOKENDAI)
Funding
KAKENHI, AMED BINDS, NIPS Joint ResearchRelease Source
Title: Acid-stable capsid structure of Helicobacter pylori bacteriophage KHP30 by single-particle cryoelectron microscopyAuthors: Ryosuke Kamiya, Jumpei Uchiyama, Shigenobu Matsuzaki, Kazuyoshi Murata, Kenji Iwasaki, and Naoyuki Miyazaki
Journal: Structure
Issue: S0969-2126(21)00329-4. Epub ahead of print.
Date: Sep. 27, 2021
URL: https://doi.org/10.1016/j.str.2021.09.001

