Structural determinants of M2R involved in inhibition by Sigma-1R
2024.12.16
Research
Abstract
Sigma-1 receptor (S1R) is a multimodal chaperone protein that is implicated in various pathophysiological conditions including drug addiction, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS). S1R interacts with various ion channels and receptors on the endoplasmic reticulum or plasma membrane (PM). It has been reported that S1R colocalizes with the M2-muscarinic acetylcholine receptor (M2R) on the soma of motoneurons, although a functional interaction between these two proteins has not been established. Here, we investigated the regulation of M2R signaling by S1R using electrophysiological recordings of GIRK currents in HEK293T cells. We observed that S1R strongly inhibited M2R-mediated activation of GIRK1/2, but the disease mutant linked to ALS, S1R E102Q, did not. The inhibitory effect of S1R was selective for M2R and wasn't seen when S1R was co-expressed with other Gi/o coupled receptors including M4R. Chimeric and mutant receptors of M2R and M4R were generated and analyzed, and this highlighted Ala401 in the transmembrane 6 domain (TM6) of M2R and Glu172 as well as Glu175 in the extracellular loop 2 regions of M2R, as essential for the inhibition by S1R. Co-immunoprecipitation confirmed the physical interaction between M2R and S1R. Immunocytochemical labeling of M2R and S1R expressed in HeLa cells, HEK293T cells, and cultured hippocampal neurons, showed clear PM expression of M2R throughout the cell which was decreased by coexpression with S1R but was still apparent. Taken together, our results show that S1R interacts with M2R to reduce both its PM expression and function, and this involves TM6 and the extracellular loop 2.
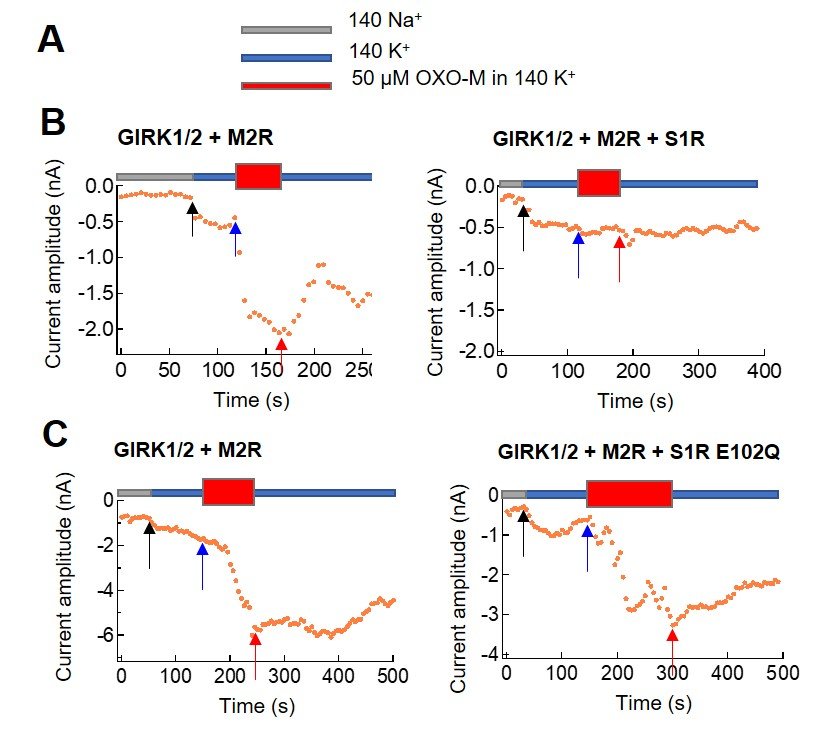
Figure 1: Analysis of the inhibition of M2R by S1R wt or mutant, monitored by GIRK current change. (A) Used external solutions. Oxo-M is a ligand of M2R. (B) GIRK current increase by activation of M2R upon Oxo-M application was inhibited by co-expression of S1R wt. (B) GIRK current increase by activation of M2R upon Oxo-M application was not inhibited by co-expression of S1R E102Q mutant.
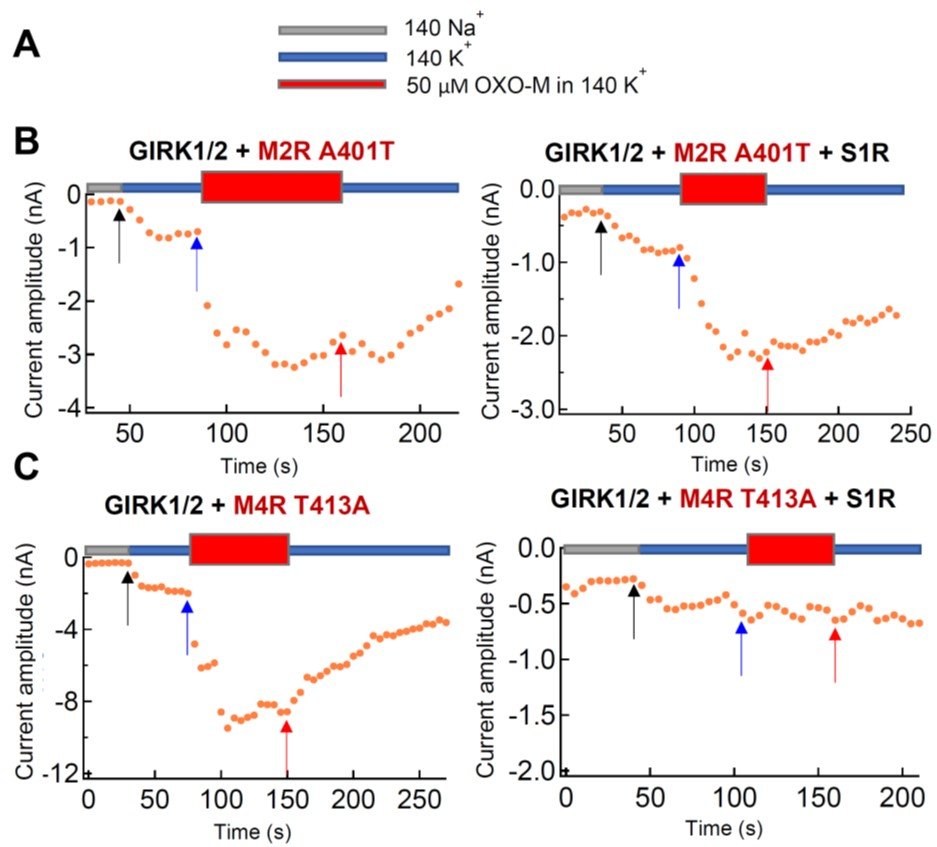
Figure 2: Analysis of the inhibition of M2R and M4R mutants by S1R wt, monitored by GIRK current change. (A) Used external solutions. Oxo-M is a ligand of M2R. (B) GIRK current increase by activation of M2R A401T mutant in the 6th transmembrane domain upon Oxo-M application was not inhibited by co-expression of S1R wt. (B) GIRK current increase by activation of M4R T413A mutant upon Oxo-M application was inhibited by co-expression of S1R wt.
Researcher
Chang Liu (NIPS Research Fellow)Yoshihiro Kubo (Professor)
Journal article
Journal of Biological Chemistry (2024) 300(12):108006Structural determinants of M2R involved in inhibition by Sigma-1R
Liu C, Chen IS, Barri M, Murrell-Lagnado R, Kubo Y.
PMID: 39551139
DOI: 10.1016/j.jbc.2024.108006.

