Introduction of Actication Study
Magnetic resonance imaging makes it possible to study the workings of the human brain in a completely non-invasive way. We will introduce this research that aims to answer the question: "Can we determine what happens in the brain during daily activities by examining changes in regional blood flow in the brain?"
Introduction
Different regions of the brain control different parts and different functions of the body. Since 1861, the localization of brain functions has been based on studies of patients with brain injury [1]. For example, visual information is processed at the back of the brain (the occipital lobe), and motor function is located in a band on the upper part of the brain. When we drink coffee, we see the coffee cup, pick it up with our hand and bring it to our mouth. This is achieved by the integration of the visual information location of the cup and the motor function of the hand. Even from this simple daily example, it is clear that in order to understand the brain, it is essential to know how the different localized functions are integrated. It is for this reason that it is important to have a non-invasive way to study the spatial distribution of neural activities and their relationships within the human brain. Such studies have been made possible by the development of noninvasive cerebral functional imaging techniques: positron emission tomography (PET); and, functional magnetic resonance imaging (fMRI). These imaging techniques are indispensable in our quest to understand higher brain functions.
Using cerebral blood flow to study brain function
Local neural activity, especially synaptic activity, increases in parallel with the glucose metabolism in a particular region of the brain. In turn, regional cerebral blood flow parallels the glucose metabolism, mediated by the oxygen supply to the region [2]. Thus, changes in local neural activity can be inferred by measuring changes in regional cerebral blood flow. Brain activation studies utilize this connection between cerebral blood flow, neural activity, and an increase in energy consumption. These methods measure the cerebral blood flow while a subject executes a particular task, and this is compared to the blood flow while the subject is relaxed and in a resting state. The distribution of differences in activity between the active and resting states is then visualized. The locations of the activity changes are thought to reflect the regions of the brain that are involved in a particular task. This principle underlies the use of cerebral blood flow to measure brain activity.
Neural activity and cerebral blood flow - historical background
The Italian physiologist Mosso [3] made the first reference to the relationship between the cerebral blood flow and neural activity. In 1881, Mosso measured the pulsation of the cerebral cortex in a patient whose cranial bone was partially missing after neurosurgery. Because this pulsation showed local increases that were simultaneous with mental activity, he concluded that the regional cerebral circulation changed following psychoneuronal activity.
In 1890, Roy and Sherrington [4] used animal studies to deduce that the increased metabolism associated with local activity in the brain causes an increase in blood flow to the location.
In 1928, Fulton [5] found that patients with arteriovenous malformation in the occipital lobe complained that they heard a murmur inside their head. This murmur, which was caused by the difference in arteriovenous blood pressure, was proportional to the blood flow. Fulton noted that this sound was stronger when the patient was reading than when they were just looking, and concluded that the regional cerebral blood flow correlated with the intensity of the mental activity. Thus, the fact that cerebral activity can be measured by changes in regional cerebral blood flow has been known for some time, but limitations in measurement techniques meant that this knowledge could be put into practice only after World War II.
In 1951, Kety [6] developed a method of quantifying the regional cerebral blood flow in experimental animals. Rapid progress in medical imaging methods since the 1970s enabled researchers to develop a non-invasive way to measure cerebral blood flow in humans.
Medical imaging techniques use electromagnetic waves to visualize the human body. Information about the inside of the body is obtained by using electromagnetic waves wavelength of which is longer (radio waves) or shorter (X-rays, gamma rays emitted by an isotopic tracer) than visible light (Table 1). Such information includes morphological and functional data; the former is found primarily by X-ray imaging and the latter by means of nuclear medicine.
Roentgen discovered X-rays in 1895 [7] and the nuclear medicine began with the discovery of natural radioactivity by Becquerel in 1896 [8]. Measurements of cerebral blood flow in humans were first made possible by nuclear medicine techniques. These techniques are based on labeling a substance with a radioisotope. The substance injected into the body then accumulates in regions of the brain in proportion to the regional blood flow, and its levels are measured from outside the body. Measurements were first conducted in the 1960s using 85Kr gas [9].
In 1972 Hounsfield invented X-ray CT [10]. The tomographical reconstruction techniques of CT were used to construct PET [11]. PET is a tomographical technique, based on the measurement of gamma rays (emitted when a positron in annihilated) and the calculation of the distribution of the positron-emitting tracer in the body. By using the appropriate tracer, various physiological and biochemical measurements can be conducted in addition to the measurement of cerebral blood flow.
|
Electromagnetic radiation used for medical imaging
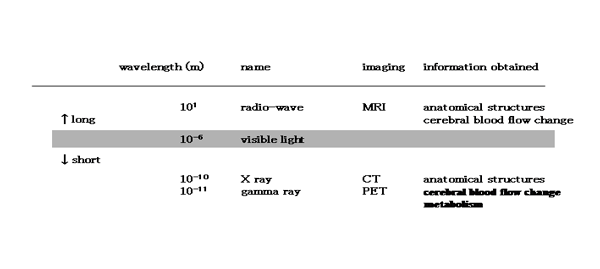
|
MRI
Compared with the medical application of short electromagnetic waves, MRI is a relatively recent technique. Information about the inside of the body is visualized using radio waves with long wavelengths. MRI is an imaging technique that utilizes the NMR of the hydrogen atom. The phenomenon of NMR was discovered, independently, by Bloch and Purcell in 1946 [12, 13], and was developed principally in the field of chemistry. A report in the early 1970s revealed that this technique was useful in differentiating between malignant and benign tumors, of great importance to medical diagnosis [14]. This yielded a prime opportunity to create medical imaging systems based on the NMR phenomenon, and, in 1973, Lauterbur invented the MRI [15].
When a hydrogen atom is placed in a uniform static magnetic field, it absorbs (resonance) and emits (relaxation) a radio wave with a specific frequency (the phenomenon of NMR). By placing a coil in parallel to the static magnetic field, this phenomenon can be detected as a gradually-decaying alternating current; this alternating current is the MR signal. The positional information embedded in this MR signal is captured based on the principles of CT (Fig. 1). The image obtained primarily reflects differences in the distribution density and the speed of relaxation of the hydrogen atoms, which in turn reflects the different composition of tissues in the body.
By changing the photographic parameters, images that emphasize various contrasts between tissues can be obtained. Compared with X-ray imaging, MRI has several advantages. First, because the radio waves have less energy than X-rays (approximately 10-12), there is less probability of MRI causing tissue damage, In addition, while X-rays are best suited to detecting heavy atoms, which are present only in small quantities in the body (for example, calcium contained in the bones), MRI is suitable for detecting hydrogen atoms, present in abundance in the body (primarily as water). For this reason, it is especially useful for imaging neural tissue, which is tightly protected by the cranial bone and the spine.
|
Principle of MRI 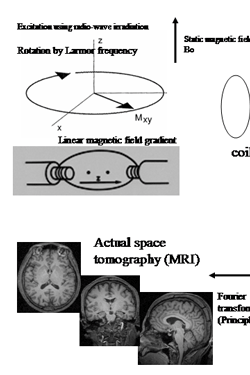 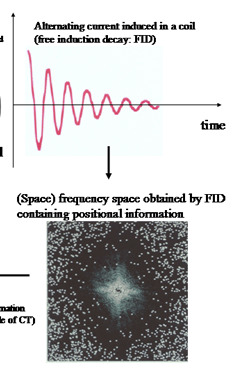 |
Principle of magnetic resonance imaging (MRI). The nuclear mag
netic resonance (NMR) phenomenon can be detected by placing a coil parallel to a static magnetic field (MR signal). In a uniform, static magnetic field, the magnetic moment created by the hydrogen atoms within the body makes a processional movement, the central axis is in the direction of the static magnetic field. Its frequency (Larmor frequency) is directly proportional to the static magnetic field in that location. If irradiated with radio waves at the same frequency, energy is absorbed and transverse magnetization (Mxy) is generated. This is detected as an alternating current that gradually decays, known as the free induction decay (FID). To obtain a tomographic image, positional information is necessary in order to separate the MR signals produced by different tissues in the body. By adding a linear gradient magnetic field to the static magnetic field, the differences between positions are expressed as differences in the magnetic fields between these locations. Since the angular velocity of a processional movement is direct proportion to the magnetic field at its location, differences in the position are reflected in differences in the angular velocity of the processional movement. Consequently, positional information is contained in the FID and is expressed as the alternating element of different angular velocities. The image obtained (bottom left) reflects the environment in which the hydrogen atoms are located and their distribution densities. Compared with X-ray computed tomography(CT), in MRI the contrast between brain tissues is higher and it can be used to obtain cross-sectional images in any direction.
Detection of regional cerebral blood flow by MRI-fMRI
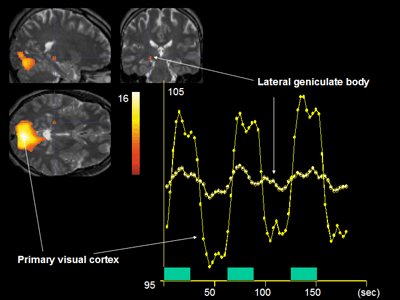
Because of the high-contrast resolution of MRI, its initial clinical application was to image the anatomical details of the brain. At the beginning of the 1990s, however, the visualization of changes in regional cerebral blood flow was made possible through the use of blood oxygen as an endogenous contrast medium, and this paved the way for fMRI [16], which is called a blood oxygen level dependent (BOLD) method, because it principally enhances small signals produced by local changes in the balance of intravascular blood oxygenation that appear during enhanced neural activity. It has long been known that oxyhemoglobin and deoxy-hemoglobin have different magnetic properties [17], and the presence of deoxy-hemoglobin in the blood vessels produces a local patchiness of the perivascular magnetic field. The uneven local magnetic field causes the NMR signal to decrease when compared to the signal in a smooth magnetic field. During enhanced neural activity there is an increase in cerebral blood flow, supplying oxygen surpassing the local demand of the neural tissue, and that, in turn, lowers the local deoxy-hemoglobin level. As a result, the NMR signal is augmented (Table 2) [16]. The advantage of this method is that changes in the cerebral blood flow to the entire brain can be recorded at intervals of a few seconds, thereby providing much more data than PET. At present, changes in the regional cerebral blood flow can be measured every second, with a spatial resolution of a few mm (Fig. 2). There is therefore high demand development of a method capable of processing complex data with improved spatial and temporal resolution.
|
The relationship between cerebral blood flow signals of PET and functional MRI
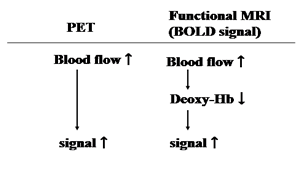 |
|
Functional magnetic resonance imaging of brain activity using photostimulation. The neural activity in the lateral geniculate body and the primary visual cortex is captured.
 |
Test example
Because of the loss of visual input in blind people, the visual cortex is no longer used for its original purpose. It is unclear, however, which functions the visual cortex continues to perform. In Braille reading, simple tactile information has to be converted into a meaningful pattern of words. Although the tactile information of Braille may be processed in a somatosensory area, letter recognition usually takes place in the visual system. In order to determine the neural network underlying Braille reading in blind people, brain activation tests using PET were conducted [18-20]. Of the eight subjects studied, two had congenital blindness and the remaining six lost their eyesight at an early age (acquired blindness). The subjects were all proficient Braille readers. The subjects were asked to read an 8-character Braille row, presented every 2.5 s, and to determine whether or not the characters formed a meaningful word. As a control, 10 subjects with normal eyesight and six blind subjects performed a non-Braille tactile discrimination task while undergoing brain imaging. During Braille reading, the occipital lobe, including the primary visual cortex, was activated in the blind subjects. Moreover, when the entire brain was observed, the results showed that the activation spread from the primary visual cortex to the parietal lobe and the dorsal side of the occipital lobe. When the blind and normally sighted subjects performed the same non-Braille tactile discrimination task, the blind subjects showed activation in the ventral side of the occipital lobe, which includes the primary visual cortex, and the secondary somatosensory area was suppressed. In the subjects with normal eyesight, exactly the opposite pattern was seen; (i.e., the ventral side of the occipital lobe was suppressed and the secondary somatosensory area was activated) [19]. These results were revalidated using fMRI (Fig. 3). However, it was unclear whether this dramatic functional restructuring was age-dependent or not. To answer this question, 15 blind people, who lost their eyesight at various ages and were all proficient Braille readers, were used as subjects in a brain activation study using fMRI [20]. Because of the high volume of data obtained in fMRI, the area of the brain activated in each individual can be determined. The subjects performed a passive discrimination task using Braille. The results showed that, in the subjects who lost their eyesight before the age of 16, the primary visual cortex was activated by the tactile discrimination task, while no such activation was seen in subjects who had lost their eyesight at a later age (Fig. 4). No age-dependent activity was seen in the visual association cortex. The reason for this may be that tactile stimuli activated the visual association cortex where competitive balance between the tactile and visual modalities is weighed towards the tactile modalities in an age-independent way [20]. It has thus been shown that, as a result of the deprivation of visual input over a long period of time, the tactile discrimination information may be processed in an area other than the area that received the original input (visual cortex).
|
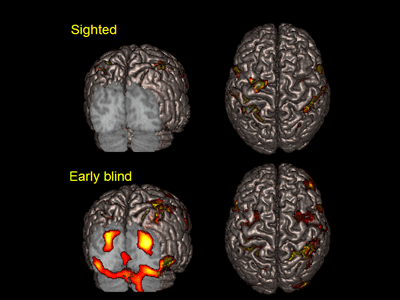
Areas activated by Braille tactile discrimination task in the sighted (top row) and the blind participant who lost his sight at 3 years old (bottom row).
The areas are superimposed on surface-rendered high-resolution MRI image viewed from back (left) and top (right).
In blind subjects, activity was seen in the occipital lobe, which includes the primary visual cortex (bottom left).
In contrast, no activation of the occipital lobe was seen in subjects with normal eyesight (top left).
Activation of the parietal lobe was seen in both cases (right).
 |
|
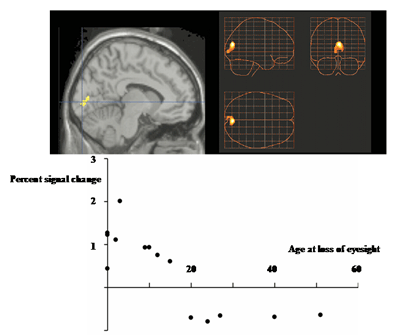
Age-dependent activation of the primary visual cortex during Braille tactile discrimination task by the blind. The change in MR signal change recorded in the primary visual cortex of the 15 blind subjects plotted against the age at onset of blindness(bottom row). Using the age of 15 as a demarcation, changes in the task-dependent activity in the primary visual cortex were studied. During the tactile discrimination task, activation was more prominent in early-onset blind, who lost their eyesight before the age of 15, than in late-onset blind participants.The focus of activation was superimposed on high-resolution MRI in the sagittal section (left, top row). The blue lines intersect in the calcarine sulcus, which is an anatomical indicator of the primary visual cortex. The three-dimensional information was collapsed into two-dimensional sagittal , coronal, and transverse images (right, top row), showing that the primary visual cortex is the only area that showed the age-dependent change.
 |
Summary
Brain-research methods can be divided into four categories [21] :
the structural analysis of the neural network, a method that includes anatomical, biochemical and molecular biological techniques;
a set of methods seeking to understand the activity inside the brain, including motor, cognitive, affective, memory, learning and autonomous functions, and their relationships to one another. This approach includes the study of brain activation, typified by functional MRI;
an approach making use of patients with brain injuries, and attempting to understand the function of damaged regions through examining particular patterns of deficits in these patients ; and,
more recently, a composition method has been developed, using theoretical simulation. The advantages of fMRI are that it can measure changes in the regional cerebral blood flow simply and repeatedly over the entire human brain and, while it can be effectively used for analyzing individual data, it can also be used for analyzing sequential data.
Finally, one of the goals of research using fMRI is to be able to integrate the processing pathways of different brain functions by understanding the relationships between regions, based on the mapping of the normal cerebral cortex in normal adults (functional localization). By making good use of these advantages, such as capturing the actual activity of the human brain, it is expected that fMRI studies will come to the forefront of the field, as they can integrate the findings of the other three approaches to studying the brain.
References
- Raichle ME. Circulatory and metabolic correlates of brain function in normal humans. In: Handbook of Physiology. Bethesda: Am. Physiol. Soc., (Mountcastle VB, Plum F, Geiger SR, ed. vol Section 1: The Nervous System. Volume V. Higher Functions of the Brain) 643-674, 1987.
- Bloch, F. 1946. Nuclear introduction. Physiol Rev 70: 460-474.
- Purcell, E.M., Torry, H.C., and Pound, R.V. 1946. Resonance absorption by nuclear magnetic moments in a solid. Physiol Rev 69: 37.
- Damadian, R. 1971. Tumor detection by nuclear magnetic resonance. Science 171: 1151-3.
- Lauterbur, P.C. 1973. Image formation by induced local interaction: examples employing nuclear magnetic resonance. Nature 243: 190-191.
- Pauling, L., and Coryell, C. 1936. The magnetic properties of and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A 22: 210-216.
- Ogawa, S., Lee, T.M., Kay, A.R., and Tank, D.W. 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87: 9868-72.
- Sadato, N., Pascual-Leone, A., Grafman, J., Ibanez, V., Deiber, M.-P., Dold, G., and Hallett, M. 1996. Activation of the primary visual cortex by Braille reading in blind subjects. Nature 380: 526-528.
- Sadato, N., Pascual-Leone, A., Grafman, J., Deiber, M.P., Ibanez, V., and Hallett, M. 1998. Neural networks for Braille reading by the blind. Brain 121: 1213-29.
- Sadato, N., Okada, T., Honda, M., and Yonekura, Y. 2002. Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuroimage 16: 389-400.
- M. Ito, Thoughts About the Brain and the Mind, Kinokuniya Bookstore, Tokyo, Japan, 1993.