Development and application of super-resolution two-photon microscopy
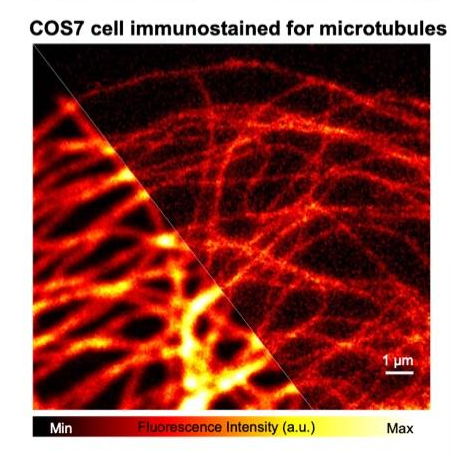
Two-photon microscopy is a powerful tool to visualize biological phenomena in the deep region of living bodies or thick tissues. It has contributed to developing various biological researches, especially neuroscience. However, due to the optical diffraction limit, conventional two-photon microscopy cannot visualize nanoscale phenomena such as morphological changes of neural dendritic spines during long-term memory formation.
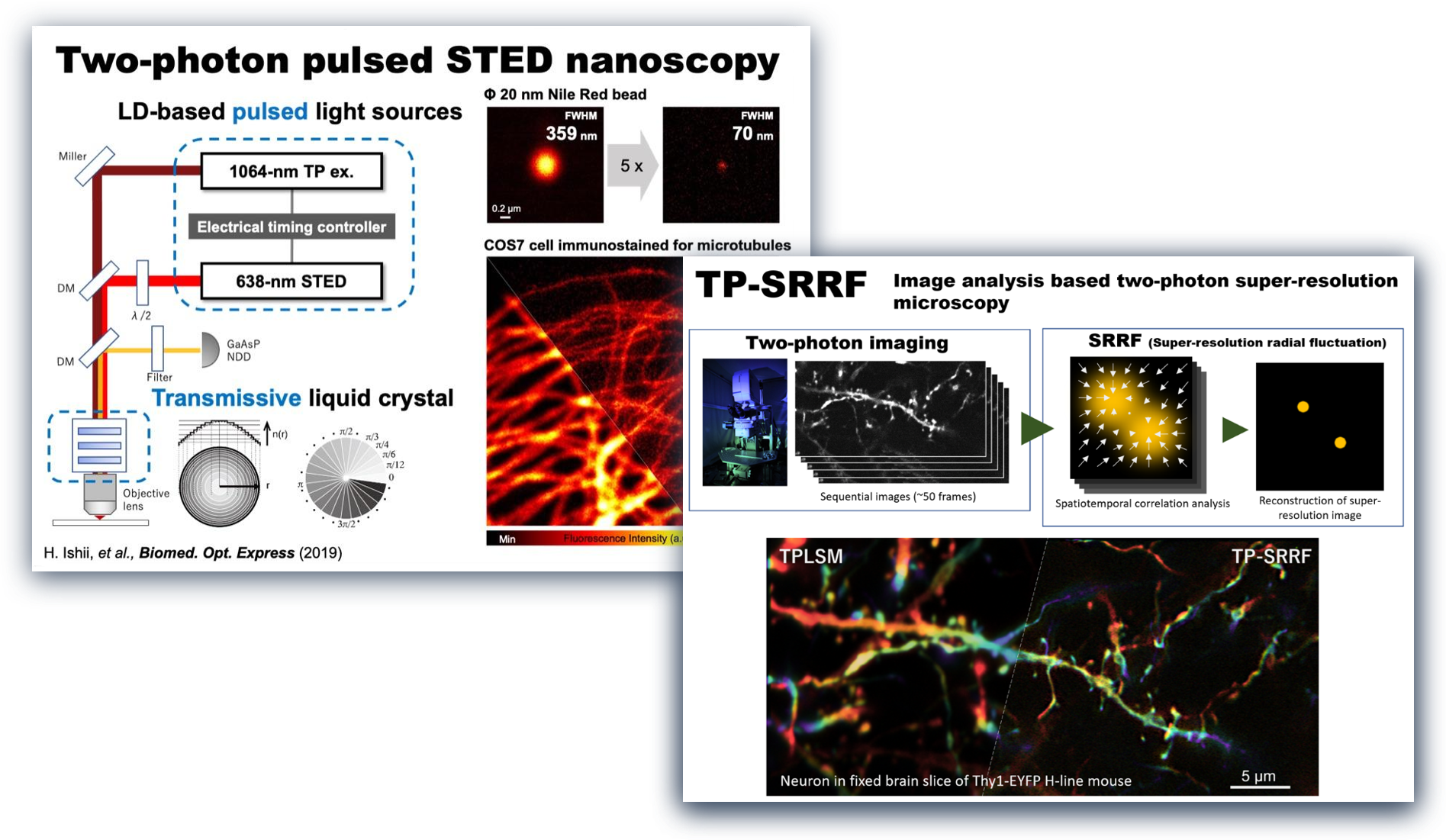
To solve such problems, we are developing super-resolution two-photon microscopies by utilizing cutting-edge optical techniques or image analyses (shown in the left Fig. and right Fig., respectively). These microscopies enable us to visualize a “real nanoworld” to uncover the mystery of life.
Press release
全パルス光源・タイムゲート検出系を駆使した超解像二光子顕微鏡の開発
~脳組織「ナノ」イメージングの新たなアプローチ~ (2023年9月4日)
https://www.excells.orion.ac.jp/news/8599
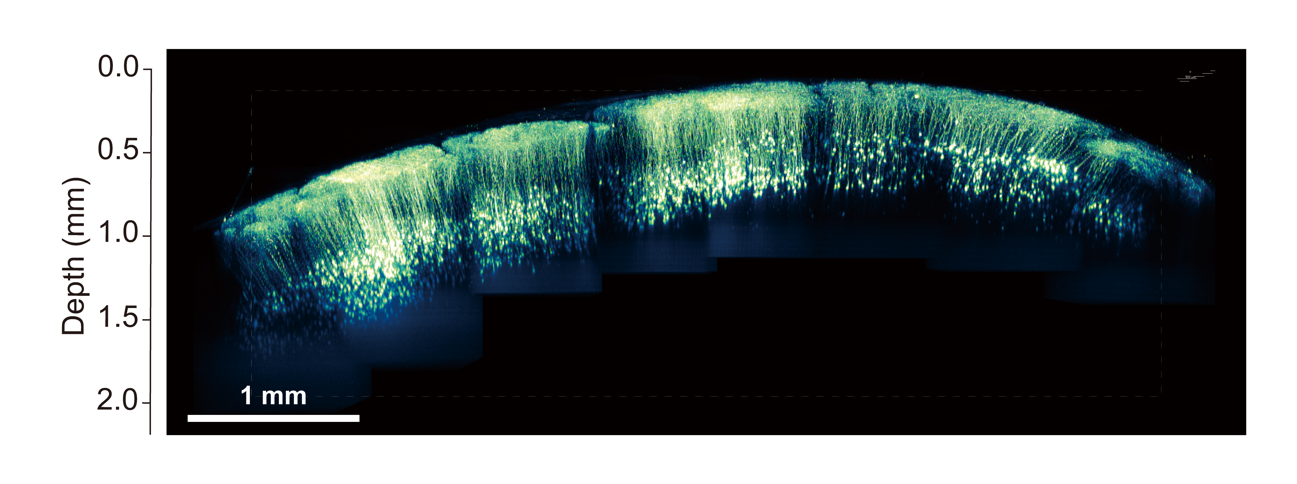
Development and application of high-speed 3D imaging methods
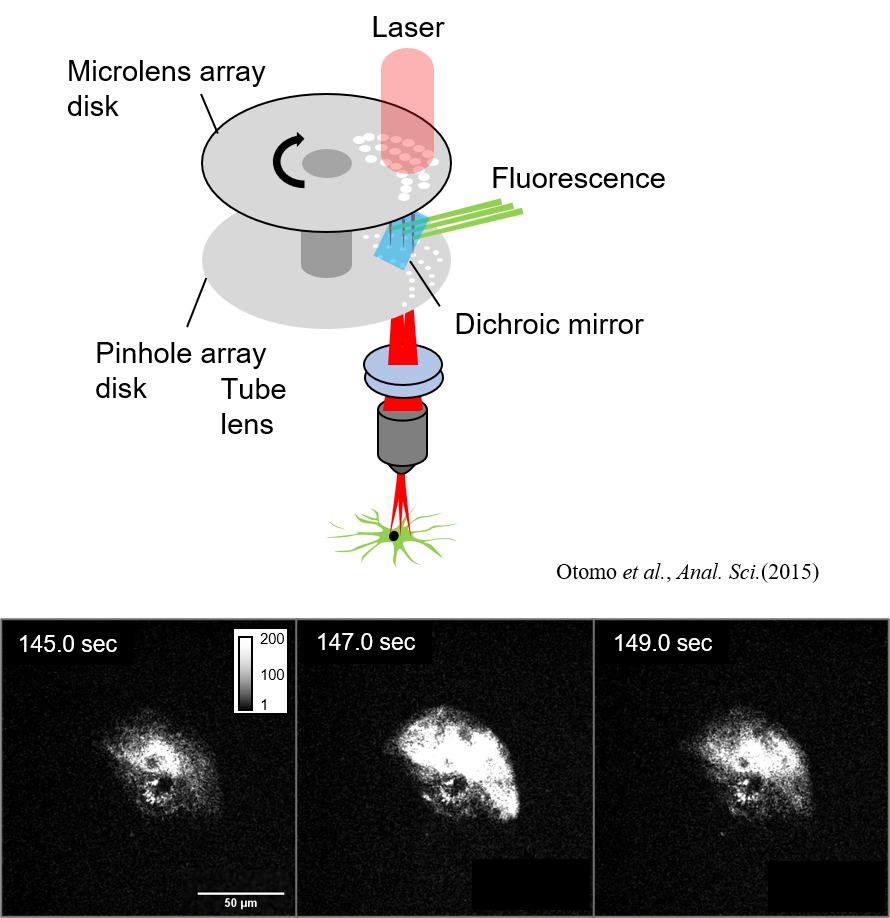
Excitation laser pulses are divided and irradiated to multiple points on the sample plane simultaneously. The resulting fluorescence is acquired with a high-sensitivity camera. Multi-point scanning using split excitation light is capable of stable 4D/5D imaging even if the number of laser pulses (repetition rate) is less than the number of pixels.
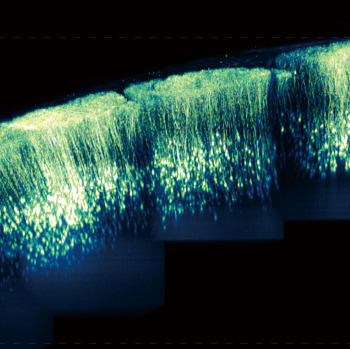
By utilizing this microscopy technique, we aim to understand living systems by visualizing microstructures inside organisms and fast calcium and glutamate responses in a wide field of view, with high spatial resolution, multicolor, and high speed. The left figure shows the results of astrocytic calcium imaging, which showed local and rapid calcium response at several locations.
Development of a method for large-scale imaging of living mouse brain utilizing novel nanosheets
In vivo two-photon deep imaging with a large field of view has revealed functional connectivity among brain regions. We developed a novel method for large-scale imaging of living mouse brain utilizing novel nanosheets. Nanosheets are thin films composed of polymers with a thickness of about 100 nm. They have unique properties of high flexibility, adhesion, and transparency. We utilized novel nanosheet to cover the entire parietal region of a mouse. By using novel nanosheet and two-photon microscopy, we have succeeded in achieving in vivo imaging of neural structure and calcium elevation in a large field of view. This research is being conducted in collaboration with Professor Yosuke Okamura at Tokai University.
T. Takahashi et al., iScience (2020) T. Takahashi et al., Communications Biology (2024)Press release
生きた動物の大脳皮質から小脳までを 高精細かつ長期的に光イメージングできる手法を開発
ー高分子ナノ薄膜と光硬化性樹脂による超広範囲な観察窓ー
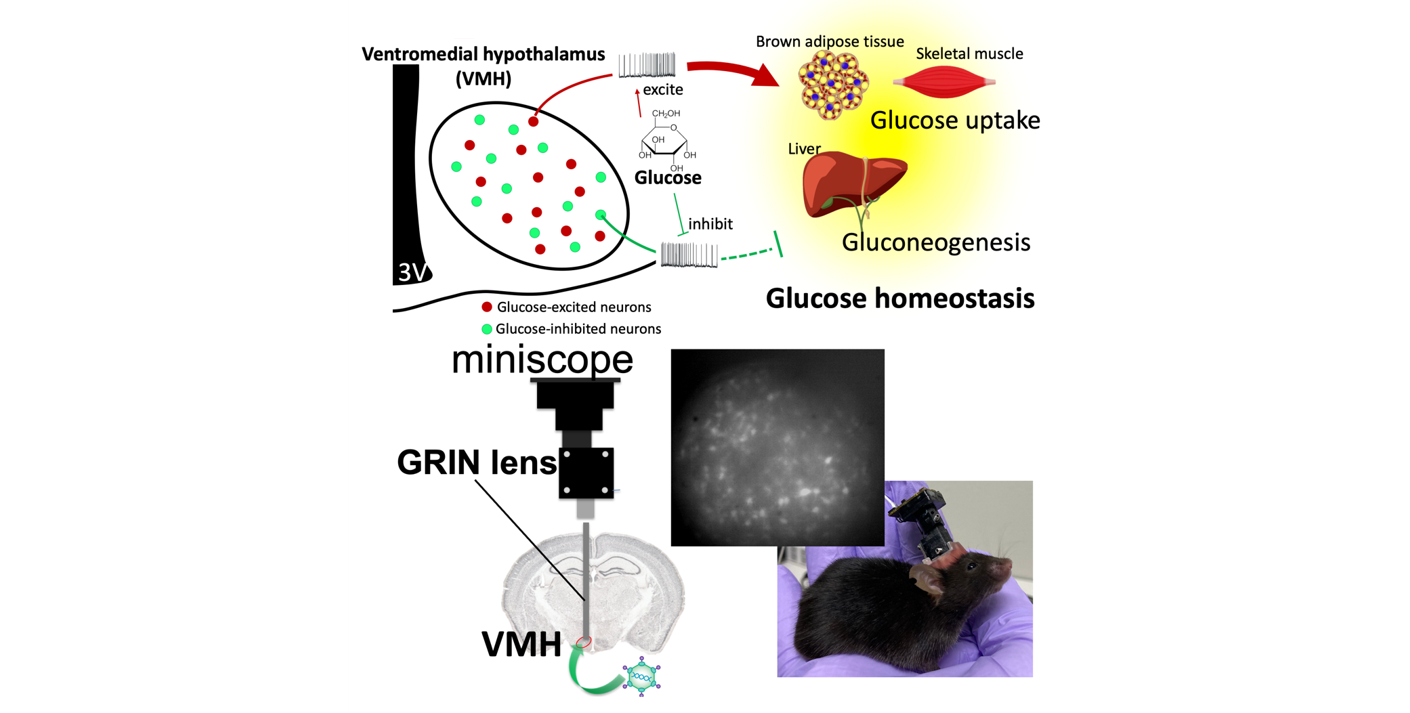
Glucose metabolism
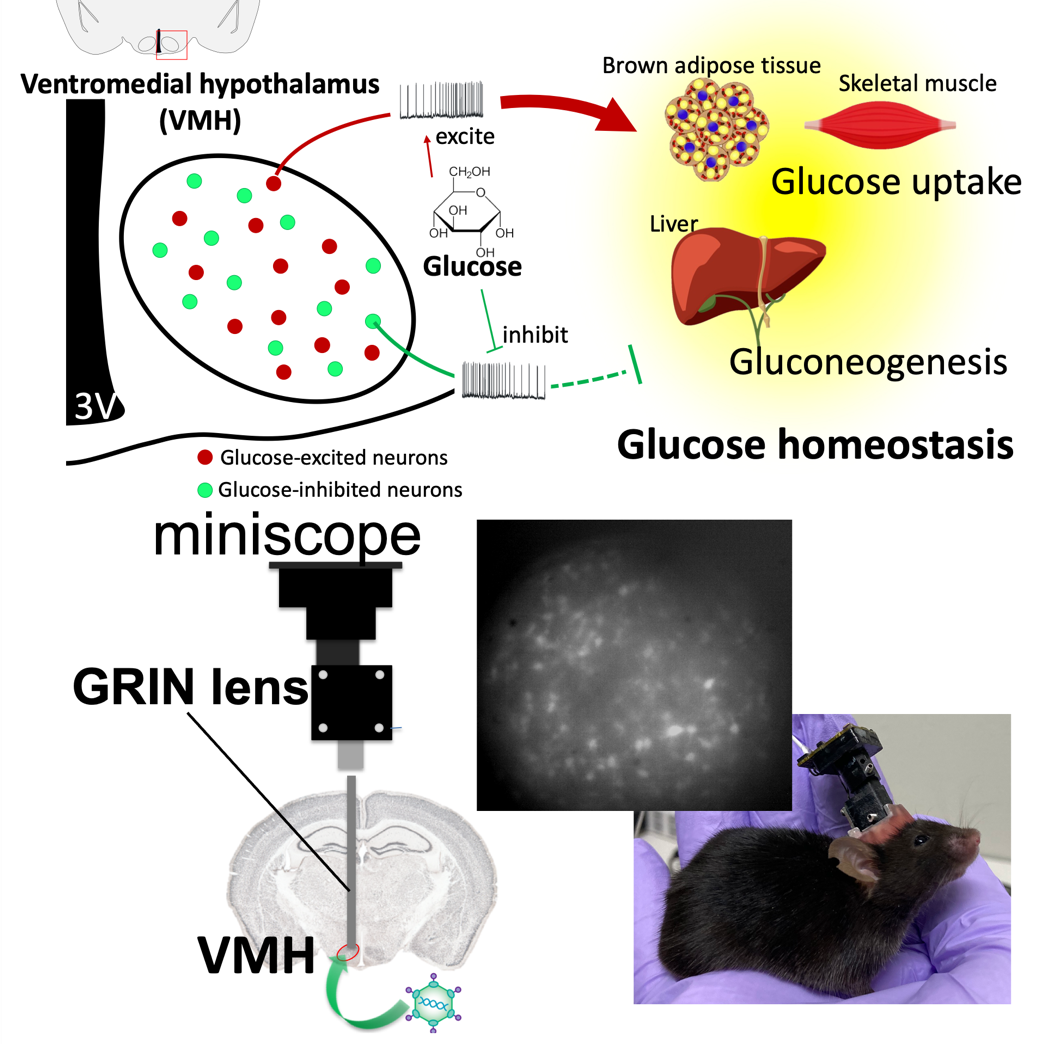
Our brain, especially the hypothalamus, plays an important role in regulating whole body glucose homeostasis. The glucose-sensing neurons in the hypothalamus, especially the ventromedial hypothalamus (VMH), detect ambient glucose and deliver the signals to other brain regions. Two types of glucose-sensing neurons, the glucose-excited (GE) neurons and the glucose-inhibited (GI) neurons are excited or inhibited by glucose, respectively. These neurons regulate peripheral glucose uptake and liver gluconeogenesis to control blood glucose. We are studying properties and roles of these glucose-sensing neurons under different situations, such as satiety, hunger or even torpor. To achieve it, we combine in vivo calcium imaging with a miniscope to observe neuronal activity in free-moving animals and the hyperinsulinemic-euglycemic clamp technique to assess systemic glucose metabolism.
ML. Lee et al., Nat. Commun. (2021)Mammalian Hibernation and Torpor
Many mammals suppress thermogenesis and metabolism during the harsh winter season when food is not available, and trigger a hypothermic and hypometabolic state. Although this phenomenon of hibernation/torpor has been known for a long time, much of the molecular and cellular mechanisms are still in mystery. We are trying to understand what is going on in the mammalian brain during hibernation/torpor by using advanced optical imaging techniques. We are interested in understanding the circadian clock, glucose metabolism, and heat-sensing/production mechanisms in the brain.
「冬眠生物学〜哺乳類の低代謝・低体温による生存戦略」 websiteCircadian rhythm
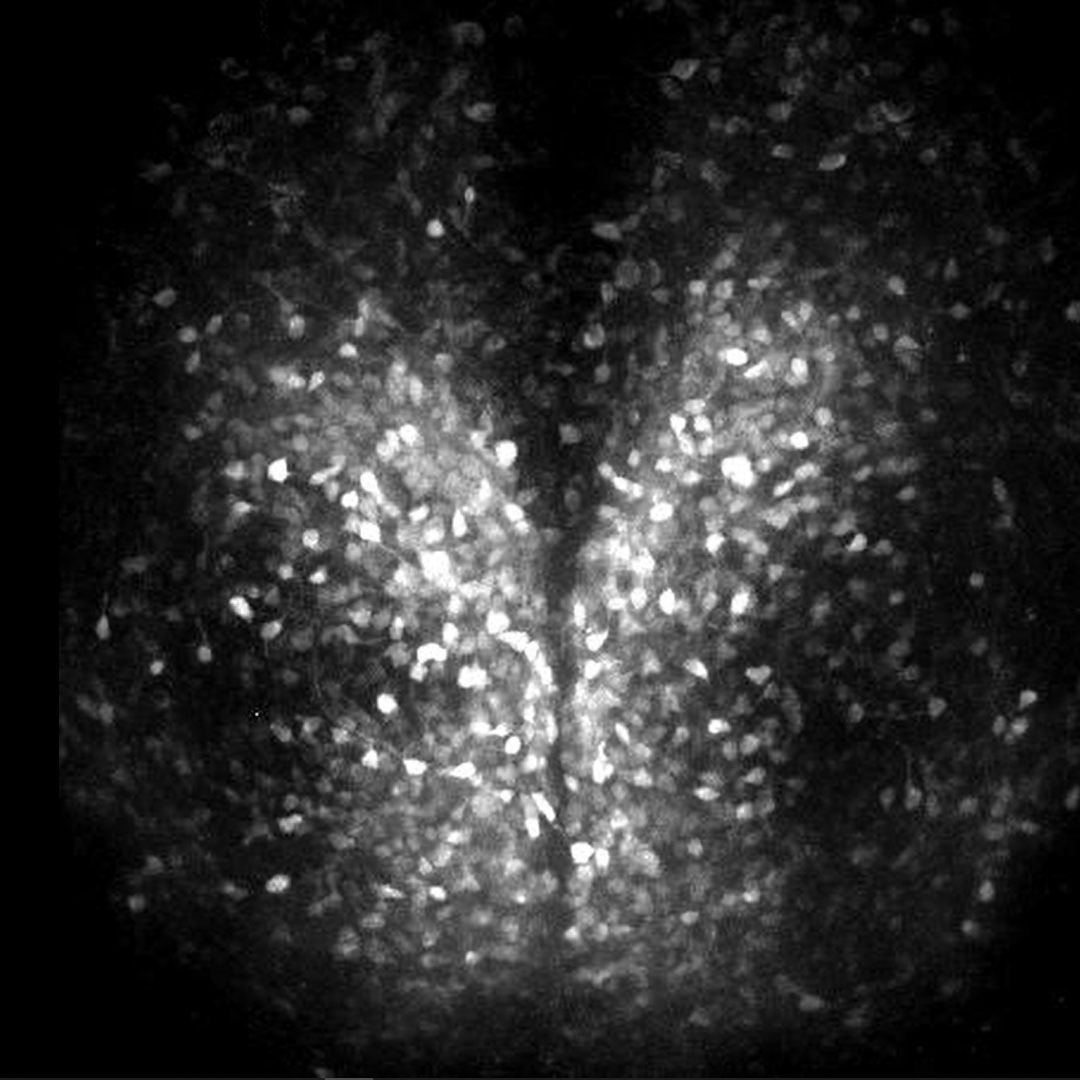
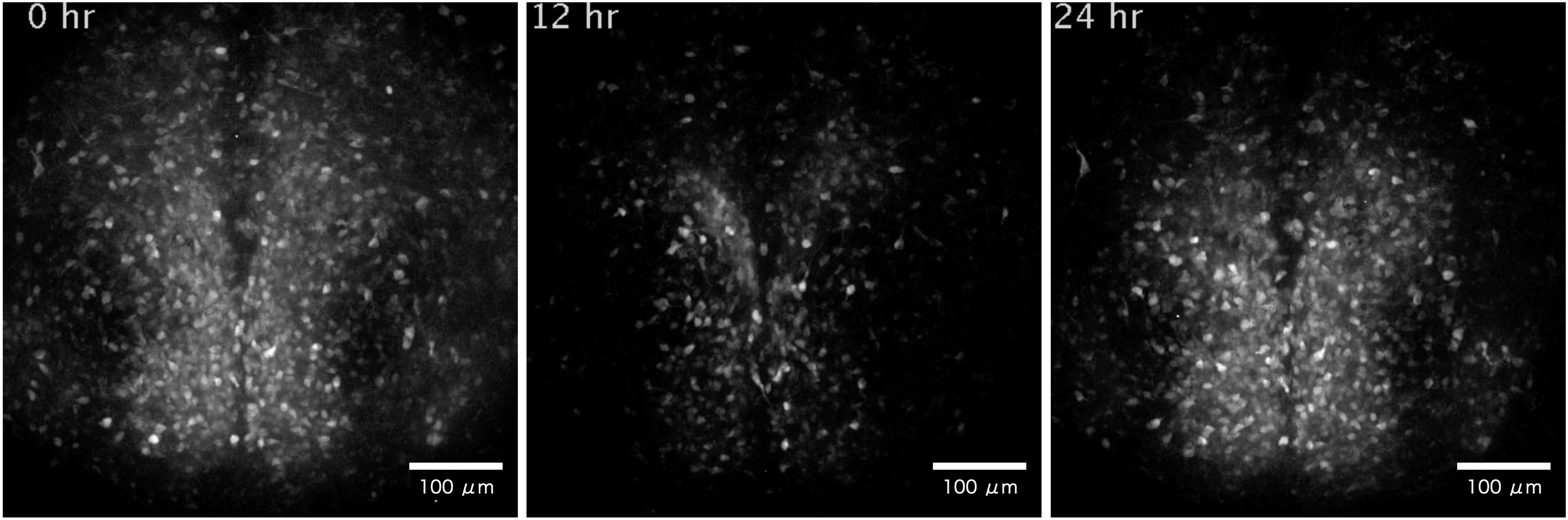
In the mammalians, the master circadian clock is located in the suprachiasmatic nucleus of the brain, where it regulates cyclical physiological functions such as the sleep-wake cycle. The suprachiasmatic nucleus consists of about 20,000 neurons surrounded by glial cells, which form a network to generate a robust rhythm. To understand the mechanism of rhythm generation and its characteristics, we have been visualizing intracellular calcium, membrane potential, etc. in the suprachiasmatic nucleus using a long-term timelapse imaging system. The figure shows the activity of the suprachiasmatic nucleus in a sliced mouse brain using a light probe that detects changes in intracellular calcium concentration.
Y. Hirata, R. Enoki et al., Sci. Rep. (2019)