Fukata Lab. Div.Membrane Physiology NIPS
Fundamental mechanisms for synaptic transmission and synaptic disorders
We will elucidate the core regulatory mechanisms for synaptic transmission and finally address the fundamental question, "How does our brain physiologically function and how is the system disrupted in brain diseases?". We have focused on the regulatory mechanisms for AMPA-type glutamate receptor (AMPAR) as AMPAR plays a central role in learning and memory formation. Based on our specific and quantitative biochemical methods, we discovered two types of AMPAR regulatory proteins: the DHHC palmitoylating enzymes and the epilepsy-related ligand/receptor, LGI1/ADAM22. So far, we have elucidated the physiological functions of these two AMPAR regulatory proteins and the implication in the pathogenesis of brain diseases such as epilepsy and limbic encephalitis, by developing new methods to screen the palmitoyl enzyme-substrate pairs and to specifically visualize the palmitoylated protein, and by integrating many methods such as super-resolution imaging, mouse genetics, and electrophysiology. We will elucidate the molecular basis in which these AMPAR regulatory proteins regulate synaptic plasticity and cognitive functions of mouse and human brains using the following our developed or cutting-edge approaches and resources.
1) Analyses of in vivo protein-protein interactions 2) Screening of palmitoylating enzyme library 3) Live cell imaging with palmitoylated protein-specific probes 4) Observation of synapses with super-resolution microscopy 5) Mouse models of human epilepsy with the LGI1 mutation
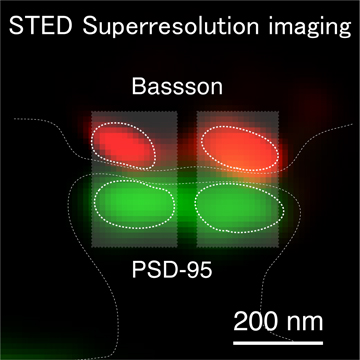
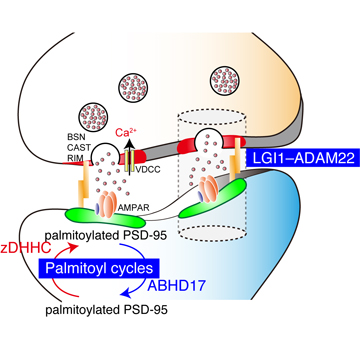
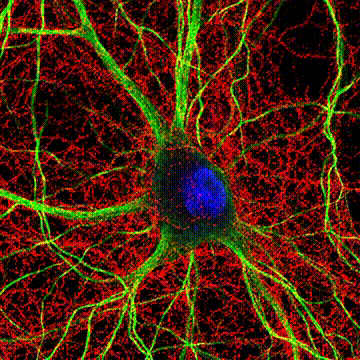
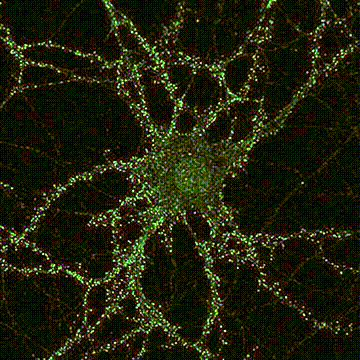
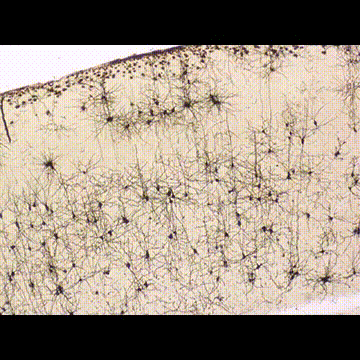
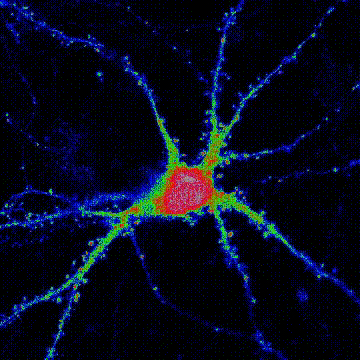
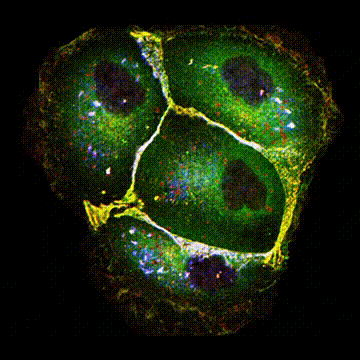
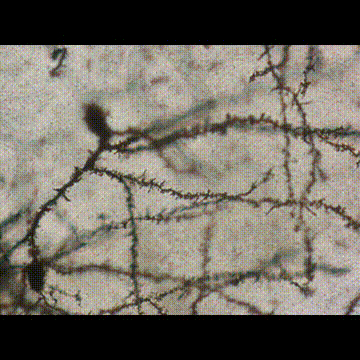
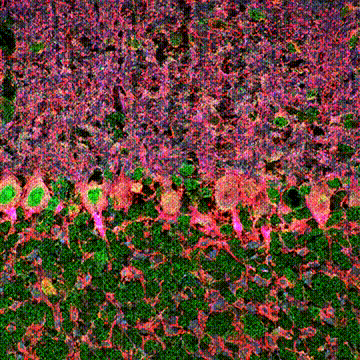
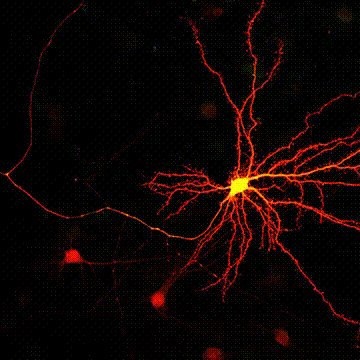

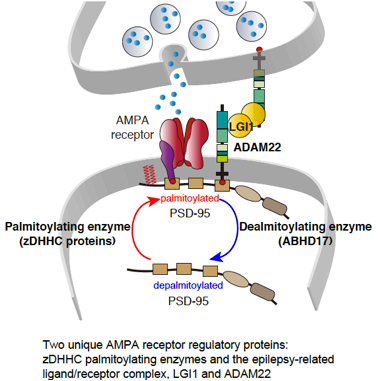
< Two unique AMPA receptor regulatory proteins: DHHC palmitoylating enzymes and the epilepsy-related ligand/receptor, LGI1 and ADAM22. >
Almuni of internships and JSP summer program
| Sultan from Turkey (2011) |
Yovita from Indonesia (2014) |
Krishna from USA (2015) |
|---|---|---|

|

|

|
| Thuy from Vietnam (2018) |
Dang from Vietnam (2019) |
|---|---|

|

|