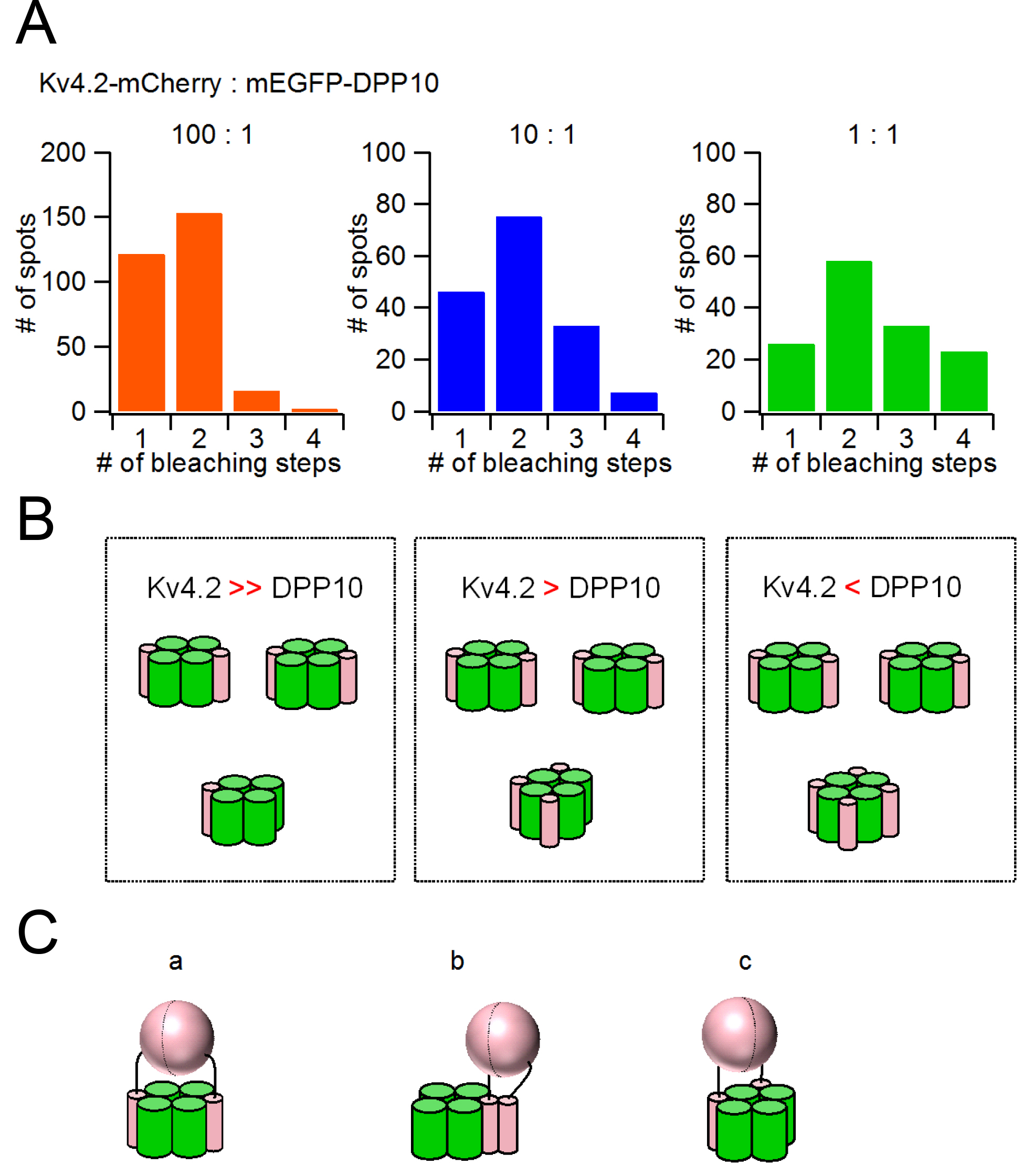
Kv4 is a member of the voltage gated K+ channel family and forms a complex with various accessary subunits. Dipeptidyl aminopeptidase-like protein (DPP) is one of the auxiliary subunits for the Kv4 channel. While DPP has been well characterized and known to increase the current amplitude and accelerate the inactivation and the recovery from inactivation of Kv4 current, it remains to be determined how many DPPs bind to one Kv4 channel. To examine whether or not the expression level of DPP changes the biophysical properties of Kv4, we expressed Kv4.2 and DPP10 in different ratios in Xenopus oocytes and analyzed the currents under two-electrode voltage clamp. The current amplitude and the speed of recovery from inactivation of Kv4.2 changed depending on the co-expression level of DPP10. This raised the possibility that the stoichiometry of the Kv4.2/DPP10 complex is variable and affects the biophysical properties of Kv4.2. We next determined the stoichiometry of DPP10 alone by subunit counting using single-molecule imaging. Approximately 70% of DPP10 formed dimers in the plasma membrane and the rest existed as monomers in the absence of Kv4.2. We next determined the stoichiometry of the Kv4.2/DPP10 complex: Kv4.2-mCherry and mEGFP-DPP10 were co-expressed in different ratios and the stoichiometries of Kv4.2/DPP10 complexes were evaluated by the subunit counting method. The stoichiometry of the Kv4.2/DPP10 complex was variable depending on the relative expression level of each subunit with a preference for 4:2 stoichiometry. This preference may come from the bulky dimeric structure of the extracellular domain of DPP10.
Masahiro Kitazawa, Yoshihiro Kubo and Koichi Nakajo (2015)
Kv4.2 and Accessory Dipeptidyl Peptidase-like Protein 10 (DPP10) Subunit Preferentially Form a 4:2 (Kv4.2:DPP10) Channel Complex
J. Biol. Chem. jbc.M115.646794. First Published on July 24, 2015, doi:10.1074/jbc.M115.646794
(A) Number of DPP10 subunits included in Kv4.2/DPP10 complex evaluated by single molecule imaging subunit counting method. Even when the relative expression level was changed, a peak was always observed at 4:2 stoichiometry. (B) In Kv4.2/DPP10 complex, their binding was not probabilistic but with a preference to 4:2。 (C) The manner of bind of DPP10 to Kv4.2 was suggested to be like the case of (a).
Division of Biophysics and Neurobiology, Department of Molecular Physiology, National Institute for Physiological Sciences
Koichi Nakajo (Present address: Department of Physiology, Osaka Medical College),
Yoshihiro Kubo