Epilepsy is a common neurological disorder that affects ca. 1% of the population, and understanding its pathophysiology is important for the prevention, suppression of its progress and treatment of the disease. In this study, we analyzed the rat brain in status epilepticus induced by kainic acid, which shows prolonged seizure activity, by using biochemical and immunohistochemical methods. Extracellular signal-regulated kinase 1/2 (ERK1/2) is activated in the brain in response to various types of neuronal excitation. However, studies examining whether its substrate phosphorylation is also increased along with ERK1/2 activation have been scarce. We have been studying ERK1/2 activity and the phosphorylation state of its presynaptic substrate protein, synapsin I in various types of seizure models and showed that synapsin I phosphorylation is indeed under control of ERK1/2 activity in vivo.
Here we examined the rat brain in status epilepticus and found that ERK1/2 activity was profoundly increased, whereas synapsin I phosphorylation was largely decreased (Fig. A). All these changes were confined during seizure activity and disappeared after recovery from it. These results indicate that synapsin I phosphorylation is dynamically regulated by the balance between kinase and phosphatase activities (Fig. B). The contrasting features of robust ERK1/2 activation yet synapsin I dephosphorylation as in this study (Fig. B, far right) may be indicative of an irreversible pathological outcome of the epileptic state in vivo.
Yoko Yamagata and Angus C. Nairn
Contrasting features of ERK1/2 activity and synapsin I phosphorylation at the ERK1/2-dependent site in the rat brain in status epilepticus induced by kainic acid in vivo.
Brain Research, 1625:314-323, 2015
doi: 10.1016/j.brainres.2015.08.023
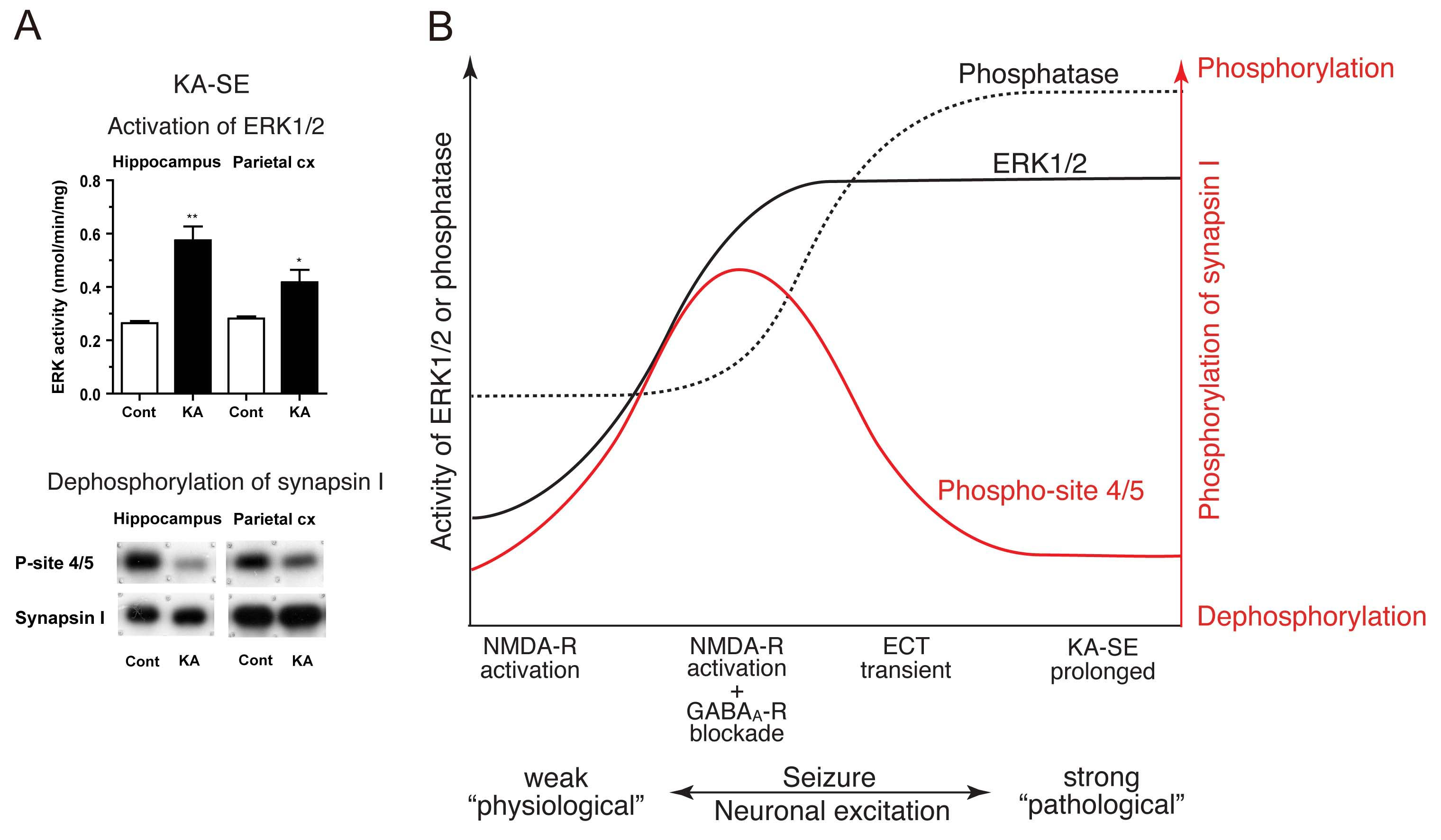
A: In kainic acid-induced status epilepticus (KA-SE), robust ERK1/2 activation occurred, whereas ERK1/2-dependent phospho-site 4/5 of synapsin I showed a large decrease in phosphorylation in the brain.
B: Combined with our previous studies (Yamagata et al., 2002, 2013), we propose a model illustrating a relationship between neuronal excitation, ERK1/2 and phosphatase (most probably calicineurin) activities and phosphorylation at phospho-site 4/5 of synapsin I in the brain during seizure activity in vivo. (1) When seizure activity is rather weak (far left), phosphatase activity increases, but ERK1/2 activity does not, resulting in dephosphorylation of phospho-site 4/5 of synapsin I. (2) When seizure activity is moderate (middle left), the increase in ERK1/2 activity exceeds that of phosphatase activity, resulting in increased phosphorylation at phospho-site 4/5. (3) When seizure activity is rather strong but transient (middle right), both phosphatase and ERK1/2 activities are increased, but due to the preceding increase in phosphatase activity, dephosphorylation of phospho-site 4/5 dominates during seizure activity. (4) When seizure activity is much stronger and prolonged (far right), the increase in phosphatase activity exceeds that of ERK1/2 activity, resulting in dephosphorylation of phospho-site 4/5. KA-SE corresponds to this condition.