The maxi-conductance anion (Maxi-Cl) channel is ordinarily inactivated or closed in resting cells but become activated in response to a variety of biologically relevant stimuli under physiological and pathological conditions. Activated Maxi-Cl channels transport inorganic anions (mainly Cl-) thereby controlling the membrane potential, cell volume and fluid transport. Maxi-Cl channels are also known to release ATP4- and MgATP2- (effective diameter 1.1~1.3 nm) through their pore-like narrowest part (diameter 1.1~1.5 nm). Released ATP act as an extracellular autocrine/paracrine alarm signal which stimulates the purinergic receptor on the cell and/or neighboring other cells (Fig. 1). However, the molecular entity of Maxi-Cl channel has long been elusive.
Here, we demonstrated that SLCO2A1 (solute carrier organic anion transporter family, member 2a1) is an essential core (pore) component of the ATP-release Maxi-Cl channel by the following experimental data: ① By subjecting proteins isolated from bleb membranes of C127 cells rich in Maxi-Cl channel activity to proteomics analysis, 15 multiple transmembrane-spanning proteins were found to be expressed. Among them, SLCO2A1 was selected by siRNA screening. ② Heterologous overexpression of SLCO2A1 elicited Maxi-Cl channel activity in HEK293T cells that lack both endogenous Maxi-Cl channel activity and SLCO2A1 expression. Recombinant SLCO2A1 reconstituted in proteoliposomes also reproduced Maxi-Cl activity. ③ In contrast, overexpression of SLCO2A1 mutants associated with a hereditary disease of pachydermoperiostosis or primary hypertrophic osteoarthropathy failed to express Maxi-Cl channel activity. ④ The charge-neutralized SLCO2A1mutants which impair the prostaglandin transporter function became weakly cation selective with exhibiting reduced single-channel conductance.
In addition, here, we demonstrated that SLCO2A1 provides the pathway for ATP release not only from swollen cultured cells but also from mouse hearts subjected to ischemia-reperfusion (Fig. 2) by the following experimental results: ① SLCO2A1silencing in vitro suppressed the swelling-induced release of ATP from C127 cells. ② Swelling-induced ATP release was augmented by heterologous overexpression of SLCO2A1 in HEK293T cells that lack endogenous Maxi-Cl channel activity and SLCO2A1 expression. ③ However, such enhancement of ATP release was not observed when a disease-causing mutant, G222R, was overexpressed in HEK293T cells. ④ The ischemia-reperfusion insult is known to trigger ATP release from hearts. SLCO2A1 silencing in vivo suppressed the release of ATP from Langendorff-perfused mouse hearts subjected to ischemia-reperfusion.
SLCO2A1 is known as a prostaglandin transporter (PGT). In the present study, three known PGT blocker were found to inhibit Maxi-Cl channel currents and swelling-induced ATP release. Also, the charge-neutralized SLCO2A1 mutants which are known to impair the prostaglandin transporter function were found to suppress Maxi-Cl channel currents. These results suggest that SLCO2A1 functions in two modes: as a prostaglandin transporter in the resting state and as a Maxi-Cl channel in the activated state under hypotonic or ischemia-reperfusion stress and that PGE2, ATP and Cl- share the same pathway (Fig. 3).
The molecular expression of SLCO2A1 and the functional expression of Maxi-Cl channel activity have been demonstrated in multiple tissues and organs such as the brain, heart, lung, liver, gastrointestinal tract, kidney and eyes. Thus, the mechanisms of physiological and pathological functions of Maxi-Cl channels are to be elucidated on the molecular level. Since release of ATP is known to play a protective role in ischemia-reperfusion-induced heart injury, it is possible that the development of SLCO2A1 activators may be a new therapeutic strategy for preventing the damage caused by myocardial and brain infarction.
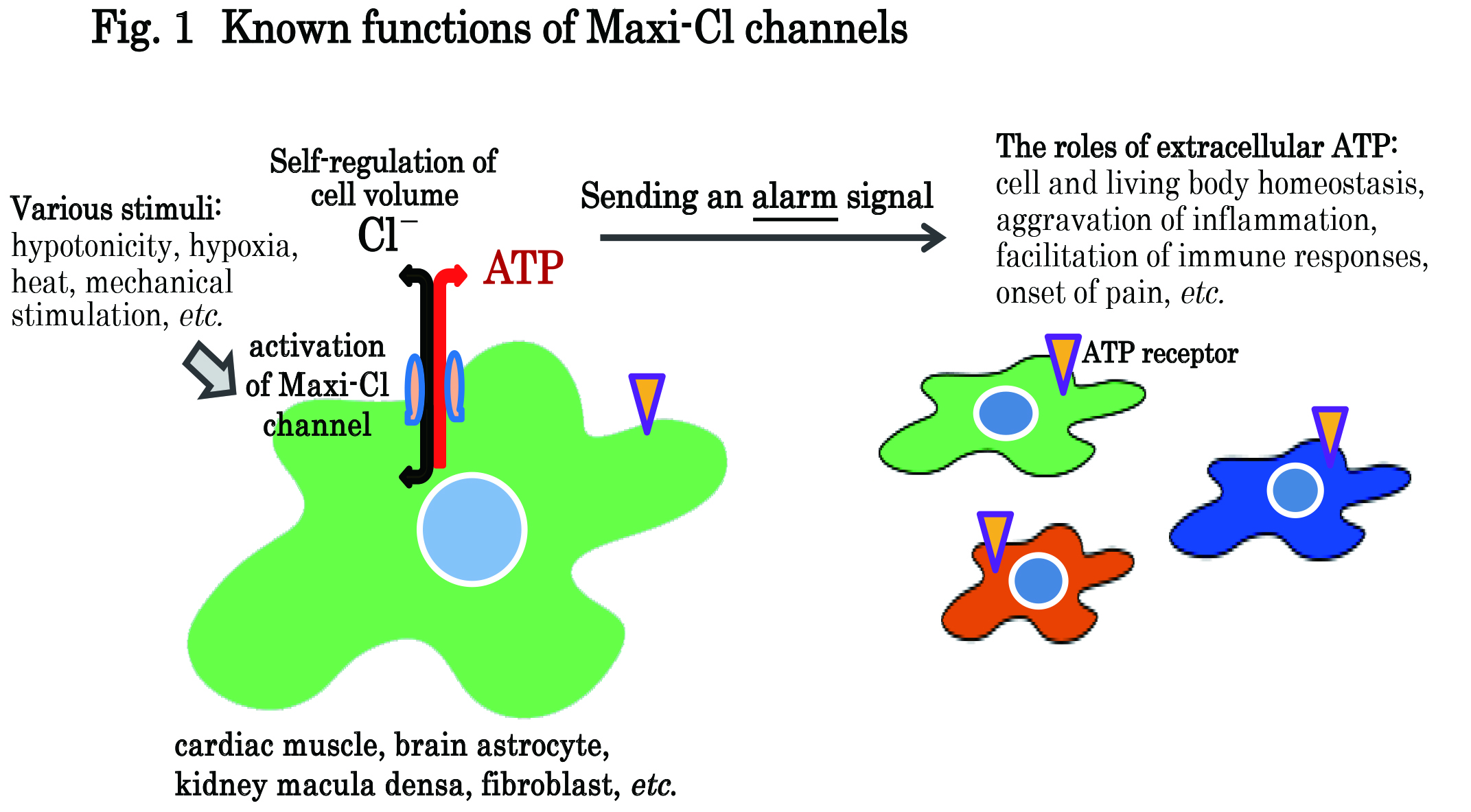
Fig 1. Maxi-Cl channels are ordinarily in the resting (closed) state but become activated to open when stimulated by hypotonic challenge, receptor stimulation or ischemia-reperfusion. Activated Maxi-Cl channels, via their pores, not only transport inorganic anions (mainly Cl-) from or to the cell but also release intracellular organic anions (especially ATP4- and MgATP2-) from the cell. Released ATP serves as an extracellular autocrine/paracrine alarm signal by stimulating purinergic receptors on the cell and neighboring other cells. Under physiological conditions, Maxi-Cl channels are involved in cell volume regulation in osmotically swollen cells by releasing the intracellular anionic osmolytes and also in promotion of cell growth, migration and differentiation by releasing ATP. ATP released from macula densa cells in the kidney provides a signal for controlling the volume and NaCl concentration of body fluids. Under pathological conditions, on the other hand, released ATP plays aggravating roles in some inflammatory and allergic diseases. Thus, ATP released via Maxi-Cl channels may exert as an alarm signal, thereby playing either beneficial or deteriorating roles depending on physiological or pathological situations. Thus, molecular identification of Maxi-Cl channel is very important to understand physiological and pathological events in the human body.
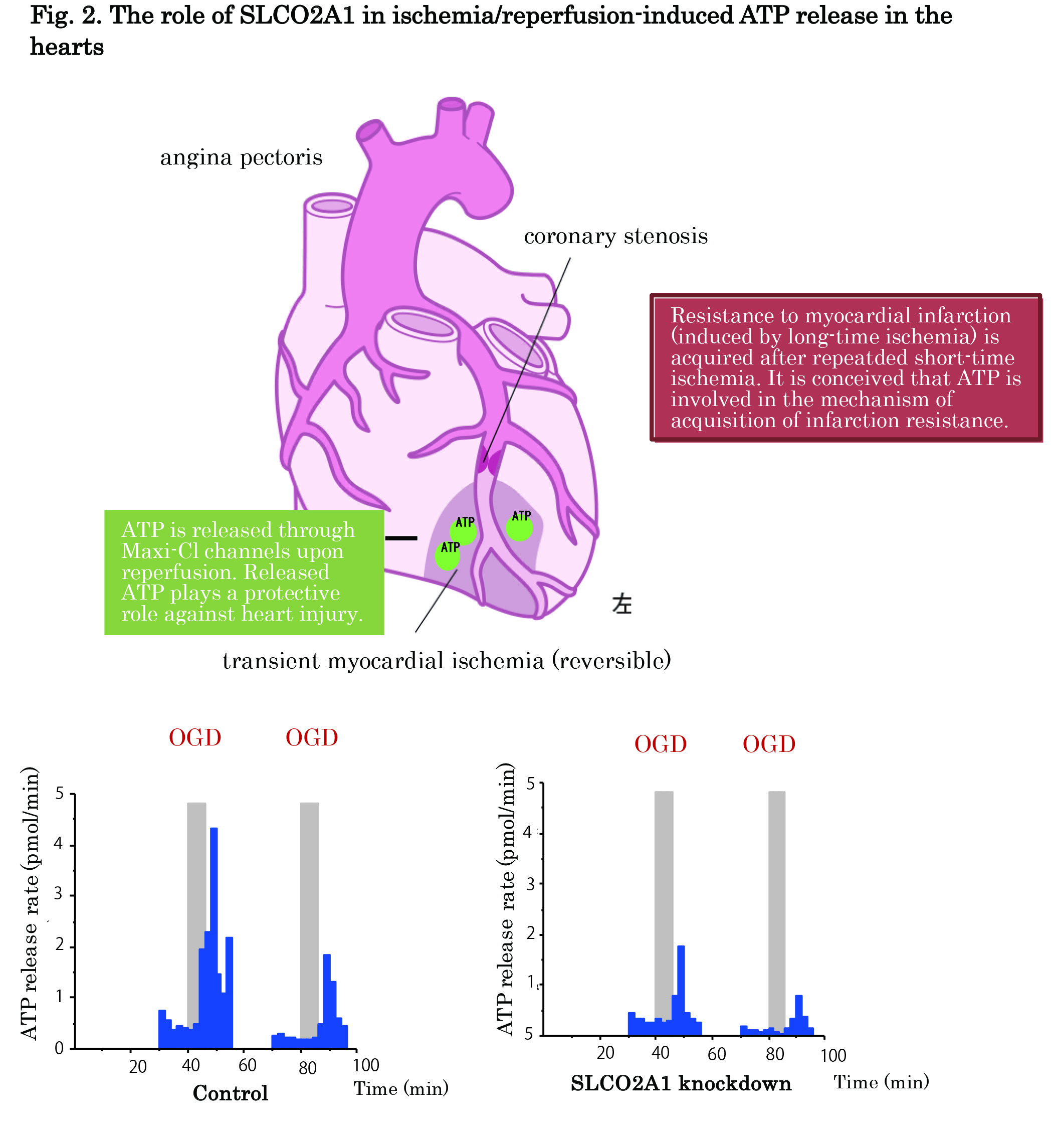
Fig 2. ATP is released (as shown as blue bars) from control mouse hearts (left graph) to the coronary effluent upon reperfusion (with oxygenated glucose-containing solution) after oxygen-glucose deprivation (OGD: at gray bars). OGD-induced ATP release was suppressed when mice were prior injected in vivo with slco2a1-tageting siRNA (right graph). Thus, SLCO2A1 is involved in the pathway for ATP release from mouse hearts in vivo upon reperfusion.
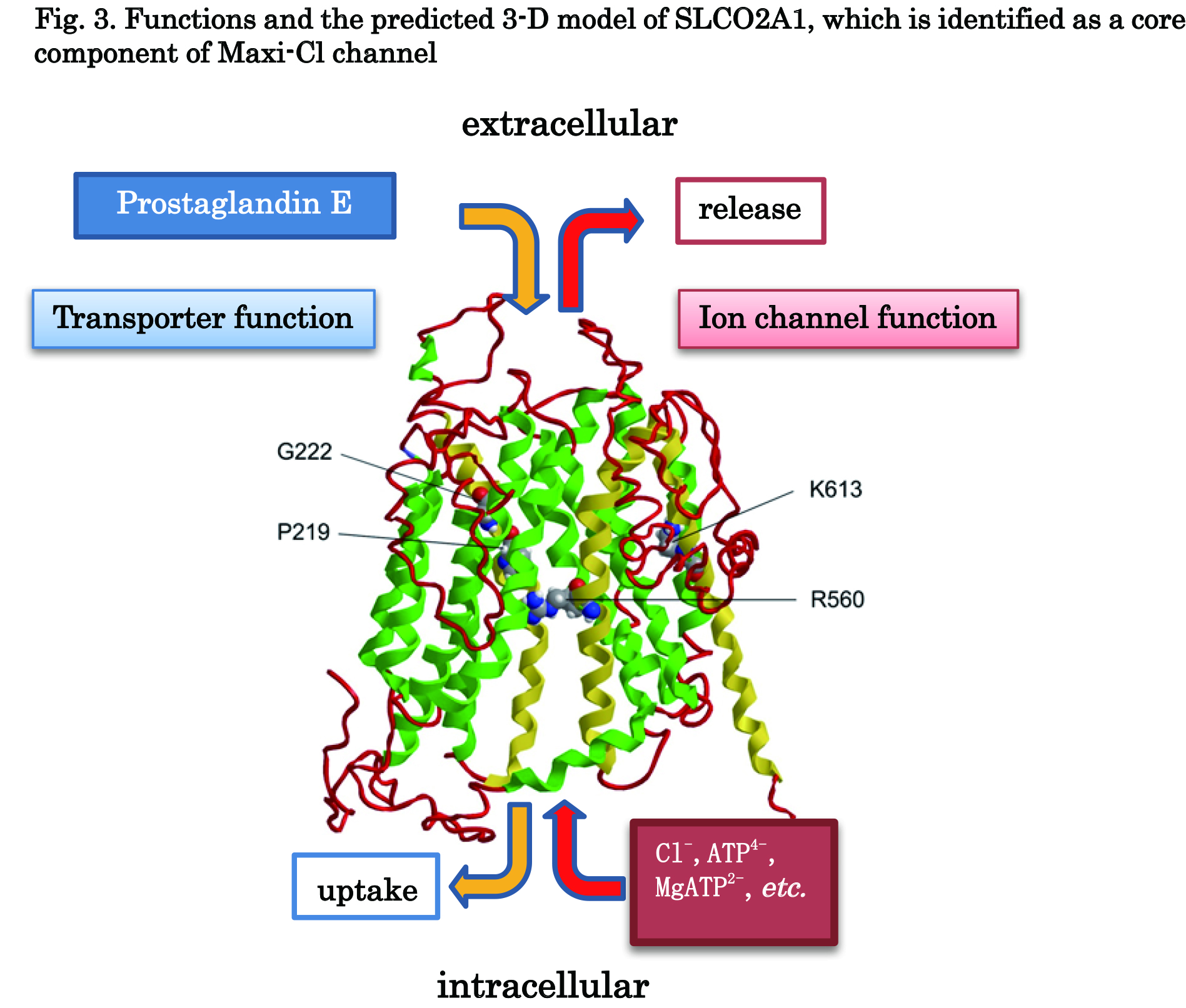
Fig 3. SLCO2A1 is known as a prostaglandin transporter (PGT) which mediates uptake of prostaglandin E2 (PGE2) into the cell. The present study showed that SLCO2A1 exhibits dual functions as a PGE transporter and a Maxi-Cl channel. This fact was supported by the experiments performed by using the disease-causing SLCO2A1 mutants, G222R and P219L, and the transporter function-impairing mutants, R560N and K613G.
This research was funded by Grants-in-Aid for Scientific Research from JSPS (#26263045, #16K08510 and #17K19517) as well as by Grants-in-Aid from the Science and Technology Agency and Academy of Sciences of Uzbekistan.
Ravshan Z. Sabirov, Petr G. Merzlyak, Toshiaki Okada, Md. Rafiqul Islam, Hiromi Uramoto, Tomoko Mori, Yumiko Makino, Hiroshi Matsuura, Yu Xie, and Yasunobu Okada.
The organic anion transporter SLCO2A1 constitutes the core component of the Maxi-Cl channel.
The EMBO Journal 2017 (in press): http://emboj.embopress.org/cgi/doi/10.15252/embj.201796685