The Epithelium separates body compartments as a barrier and selectively transports various substances, thereby contributing to organ functions and homeostasis. Our laboratory aims to clarify the molecular bases of specialized cell structures responsible for these basic roles of the epithelium. We focus on cell-cell junctions involved in the regulation of the paracellular transport (occluding junctions), including the tight junction and its related structures, and examine their molecular architectures, functions, and dynamic behavior. One of the characteristic features of our research is that we identify structural or regulatory proteins of occluding junctions and characterize their functions. We take combined approaches of molecular cell biology, physiology, and morphology, including immunoelectron and freeze-fracture replica electron microscopy, by using cultured epithelial cells and model organisms, including mice and fruit flies. The genome editing-mediated systematic loss of function experiments of relevant proteins in cultured epithelial cells is providing various new findings. The following are ongoing projects.
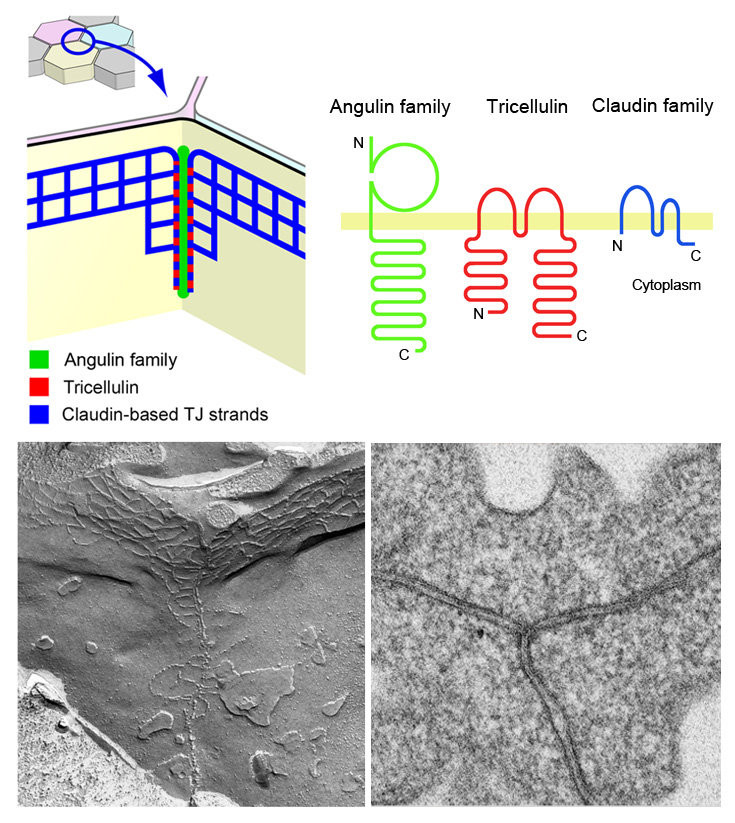 Molecular architecture and morphology of tricellular tight junctions (tTJs).
Molecular architecture and morphology of tricellular tight junctions (tTJs).
tTJs are specialized intercellular junctions formed at the point where vertices of three epithelial cells meet. tTJs contain membrane proteins angulin family proteins and tricellulin, and restrict the leakage of solutes through the intercellular space together with claudin-based tight junctions. The bottom shows a tTJ observed by freeze-fracture replica (left) and ultra-thin section (right) electron microscopy.
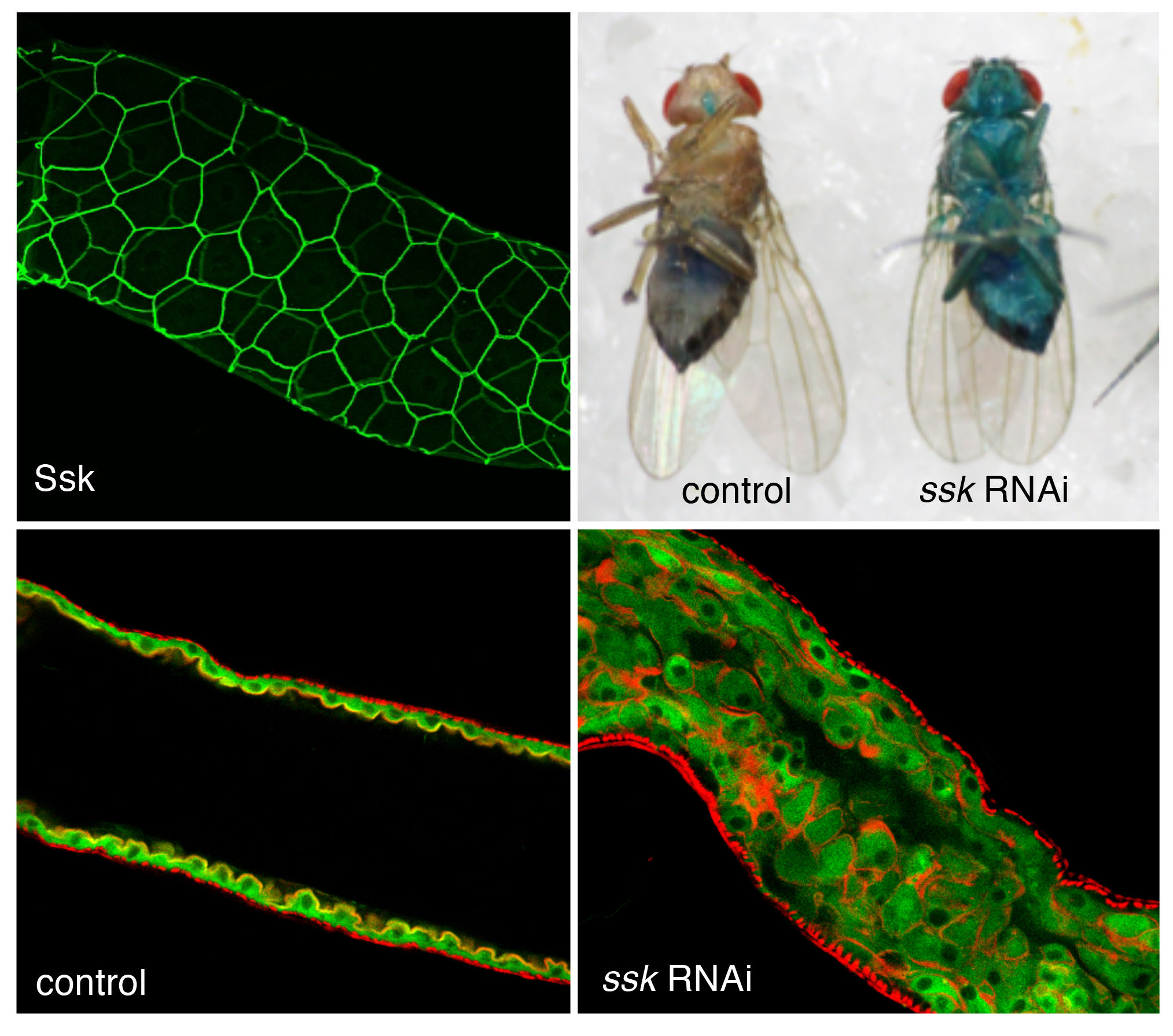
Roles of smooth septate junctions in the Drosophila midgut.
When the expression of a smooth septate junction-associated membrane protein Ssk is suppressed in the adult Drosophila gut, the intestinal barrier function is impaired, leading to the leakage of blue dye from the intestinal lumen to the body cavity with overproliferation of enterocytes.