Not only during embryonic development, but also in limited areas of the brain after birth, neural stem cells are present and continuously produce new neurons. It is becoming clear that this neurogenesis is involved in brain development and homeostasis. It has also become clear that when the brain is injured, cell proliferation in neurogenic regions increases and neurons lost due to brain injury can be regenerated. Our group, in collaboration with other research divisions of National Institute for Physiological Sciences, has elucidated the migration mechanisms of new neurons by serial block surface scanning electron microscopy (SBF-SEM) and two-photon microscopy. In this research division, we aim to elucidate the mechanisms and significance of neogenesis in the postnatal brain using normal animals and animal models of brain injury, and to use these findings to develop new therapeutic strategies.
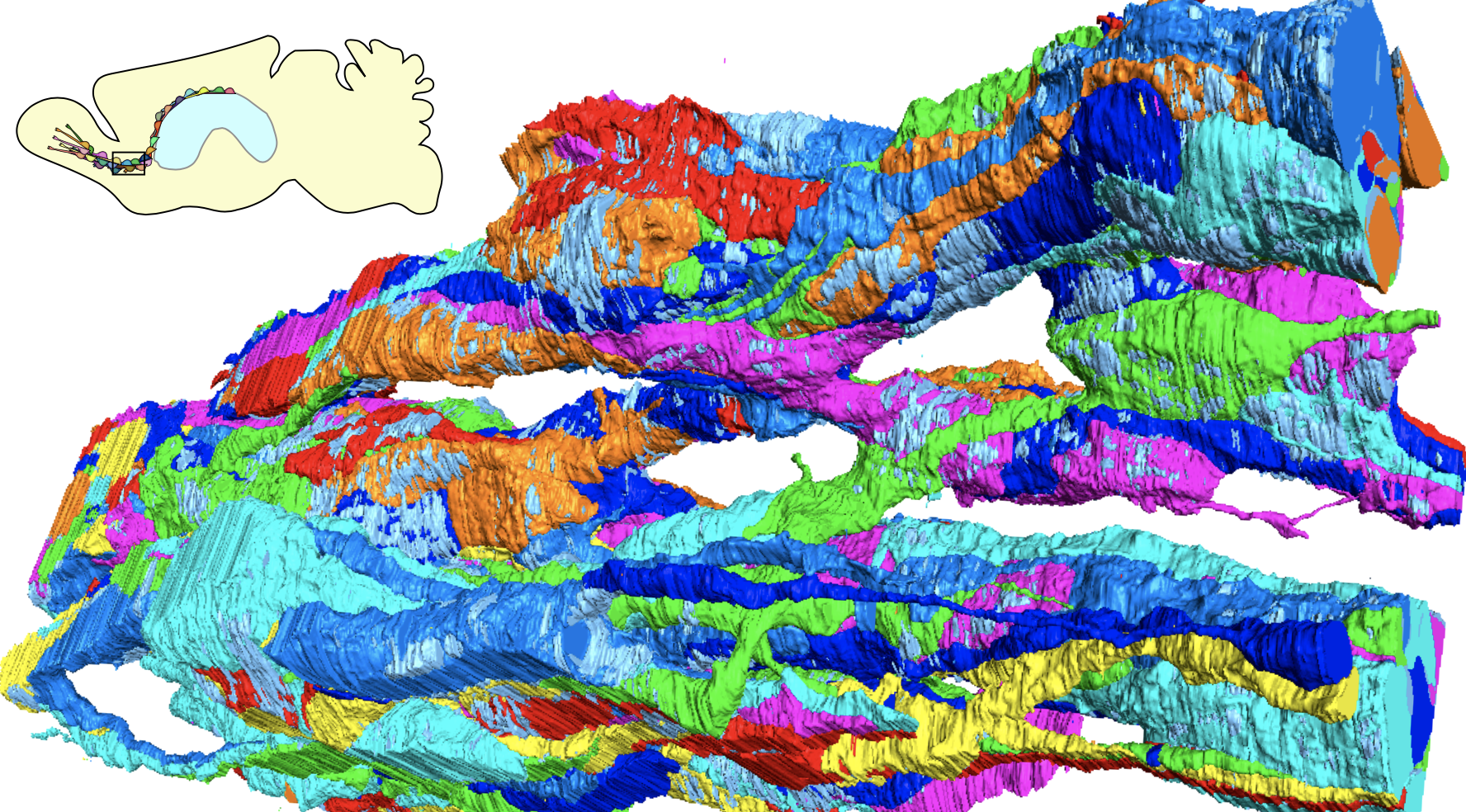 Fig.1. SBF-SEM reveals the fine structure of new neurons migrating in normal and injured brains (Matsumoto et al., 2024).
Fig.1. SBF-SEM reveals the fine structure of new neurons migrating in normal and injured brains (Matsumoto et al., 2024).
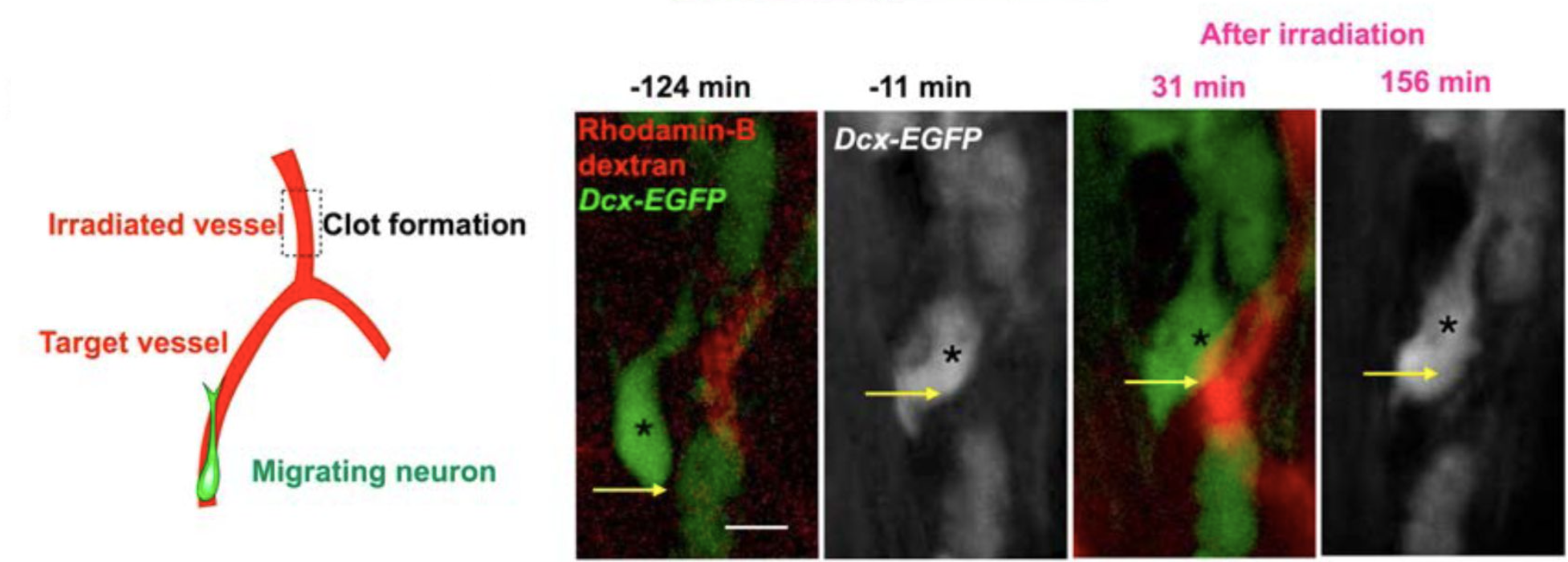
Fig.2. Two-photon microscopy reveals that the migration of new neurons is dependent on blood flow (Ogino et al., 2024).