Ion channels, receptors and G proteins play critical roles for the excitability and its regulation of neurons. We focus on these molecules which enable brain function. From the biophysical point of view, we study structure-function relationships, regulation mechanisms and dynamic structural rearrangements of ion channels and receptors. We also study the functional significance of specific features of ion channels and receptors in the brain function by making gene manipulated mice and by studying their abnormalities in the synaptic transmission and whole animal behavior.
Our experiments start with constructions of mutants, molecular chimeras and fluorescent tagged molecules of ion channels and receptors. We express them in heterologous expression systems such as Xenopus oocytes or HEK293T cells. We then analyze the functional features and dynamic structural rearrangements by electrophysiological method such as two electrode voltage clamp and patch clamp. We also use optophysiologial methods such as Ca2+i imaging, FRET analysis under total internal reflection microscope, subunit counting by single molecule imaging, and voltage clamp fluorometry using fluorescent unnatural amino acid.
Major target molecules are Two pore Na+ channel (TPC), Two pore K+ channel, G protein coupled inward rectifier K+ channel (GIRK), ATP receptor channel P2X2, Sigma-1 receptor and various G protein coupled receptors including an orphan receptor Prrt3. We also work, as cooperative research projects, on TRP channels, Opsin, as well as various ion channel toxins.
One of the characteristic features of our experimental approaches is that we utilize in vitro expression systems such as Xenopus oocytes which enable clarification of the observation targets, high through-put recordings and precise biophysical analyses by the two-electrode voltage clamp method. Another is that we perform simultaneous recordings of electrophysiology and optophysiology to approach the dynamic aspects of the function and structural rearrangements, which is beneficial towards the understanding of the functioning images. Taking advantages of these facilities and methodologies, we would like to promote our research as well as cooperative research projects further.
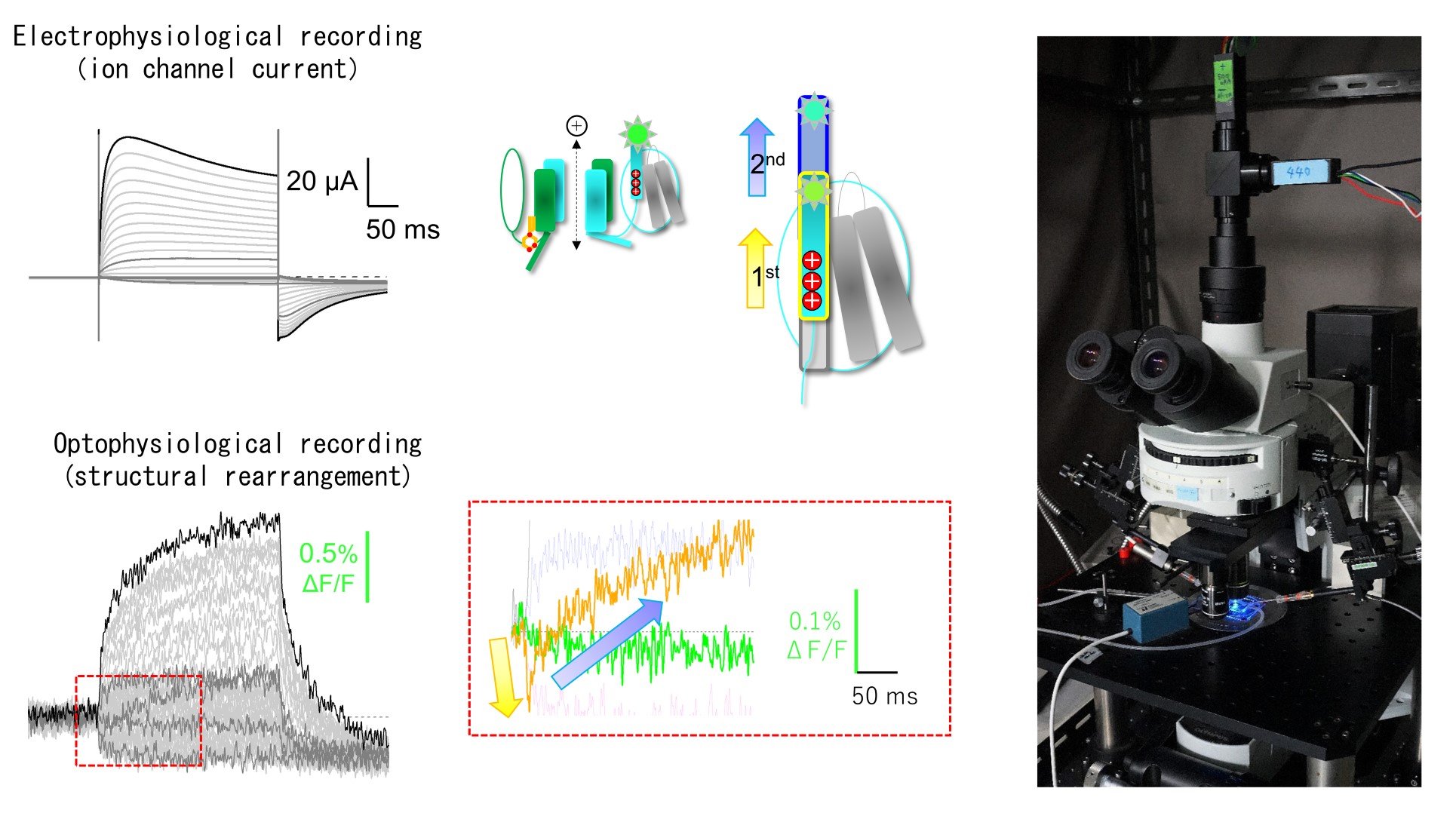
Fig. 1. Analyses of the function and dynamic structural rearrangements of TPC3 channel by simultaneous recordings of electrophysiology and optophysiology under voltage clamp using Xenopus oocyte expression systems. (Shimomura T, Hirazawa K, Kubo Y (2023) Proc Natl Acad Sci USA)