Lack of D1 receptor-mediated dopamine transmission leads to slowness of movements in Parkinson’s disease
Dopamine deficiency in the basal ganglia (a set of subcortical structures) causes severe motor dysfunctions, such as slowness of movements (bradykinesia), as observed in Parkinson’s disease. Dopamine binds D1 and D2 receptors that are expressed in the nerve cells of the striatum (a structure of the basal ganglia), and exerts different effects on the nerve cells. However, how dopamine controls through these receptors the information flow in the basal ganglia and voluntary movements is still not clear.
Assistant Professor Satomi Chiken and Professor Atsushi Nambu from National Institute for Physiological Sciences, Dr. Asako Sato from Kitasato University, Professor Toshikuni Sasaoka from Niigata University, and their research team members have revealed that lack of dopamine transmission through D1 receptors disturbs information flow through the “direct pathway” in the basal ganglia, and ends up in difficulty in initiating voluntary movements. This study was supported by JSPS KAKENHI and CREST, and published online in the Oxford journal Cerebral Cortex on October 6, 2015.


The research team successfully developed a novel transgenic mouse model in which dopamine D1 receptors can be reversibly reduced by a pharmacological agent "doxycycline", and found that the mice showed decreased movements when D1 receptors were reduced. The team used electrophysiological techniques in awake mice and examined the electrical activity of the nerve cells in the entopeduncular nucleus (EPN, the homologous structure to the internal segment of the globus pallidus in humans) that is the output station of the basal ganglia. Normally, the electrical stimulation of the motor cortex, which resembles the electrical activity during voluntary movements, causes triphasic response consisting of early excitation, inhibition and late excitation in the nerve cells of the EPN, and the "inhibition" is mediated by the “direct pathway” and acts to initiate movements. When D1 receptors were reduced in the transgenic mice by "doxycycline", the triphasic response was changed, and the "inhibition" was largely decreased. These results suggest that dopamine transmission mediated by D1 receptors is essential for information flow through the “direct pathway” to appropriately initiate movements. The research team also revealed that spontaneous activity of nerve cells in the ENP did not change when D1 receptors were reduced, which denies the prevalent view that lack of D1 receptor-mediated dopamine transmission increases spontaneous nerve cell activity in the EPN. The results suggest that transient activity changes through the “direct pathway”, not spontaneous activity changes, in the EPN are responsible for slowness of movements in Parkinson’s disease.
“We have shown that lack of dopamine transmission via D1 receptors disrupts information flow through the ‘direct pathway’ and results in slowness of movements in Parkinson’s disease. This finding provides us important clues to develop new therapies to the disease, such as on-demand activation of D1 receptors to facilitate the information flow through the 'direct pathway'”, Professor Nambu said.
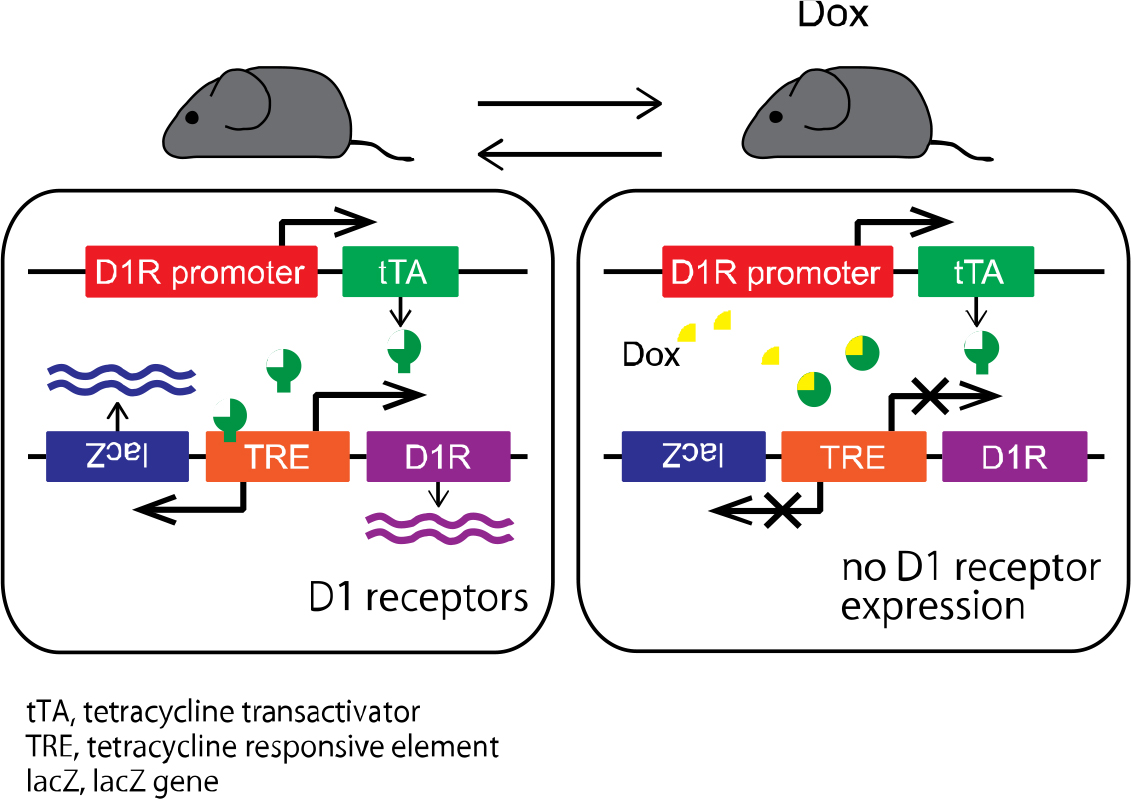
Expression of dopamine D1 receptors can be reversibly regulated by doxycycline (Dox) administration. Before Dox administration (left), D1 receptors are expressed. During Dox administration (right), the expression of D1 receptors is completely suppressed.
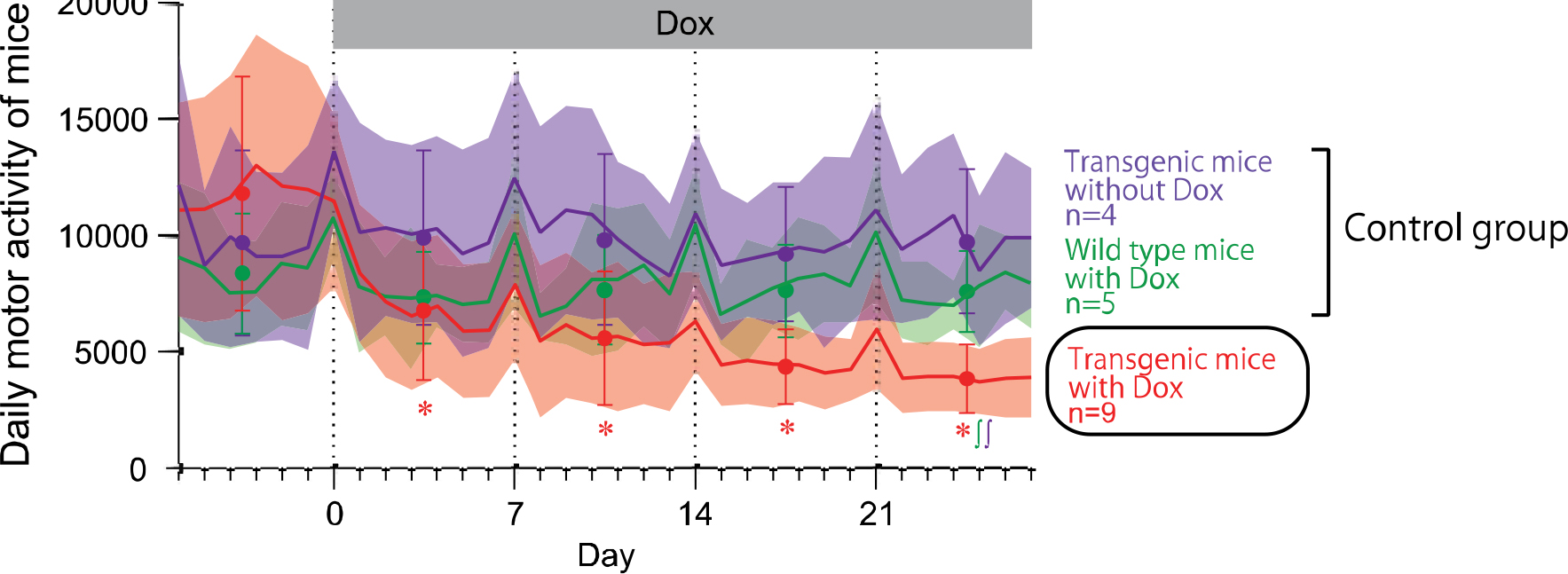
Daily motor activity of the mice in their home cages was measured before and during Dox administration. Movements of the mice started to decrease in the 1st week of Dox treatment, and monotonically decreased during the 2nd, 3rd, and 4th weeks, whereas the other control mice (transgenic mice without Dox and wild-type mice with Dox) did not show changes in daily movements during the same period.
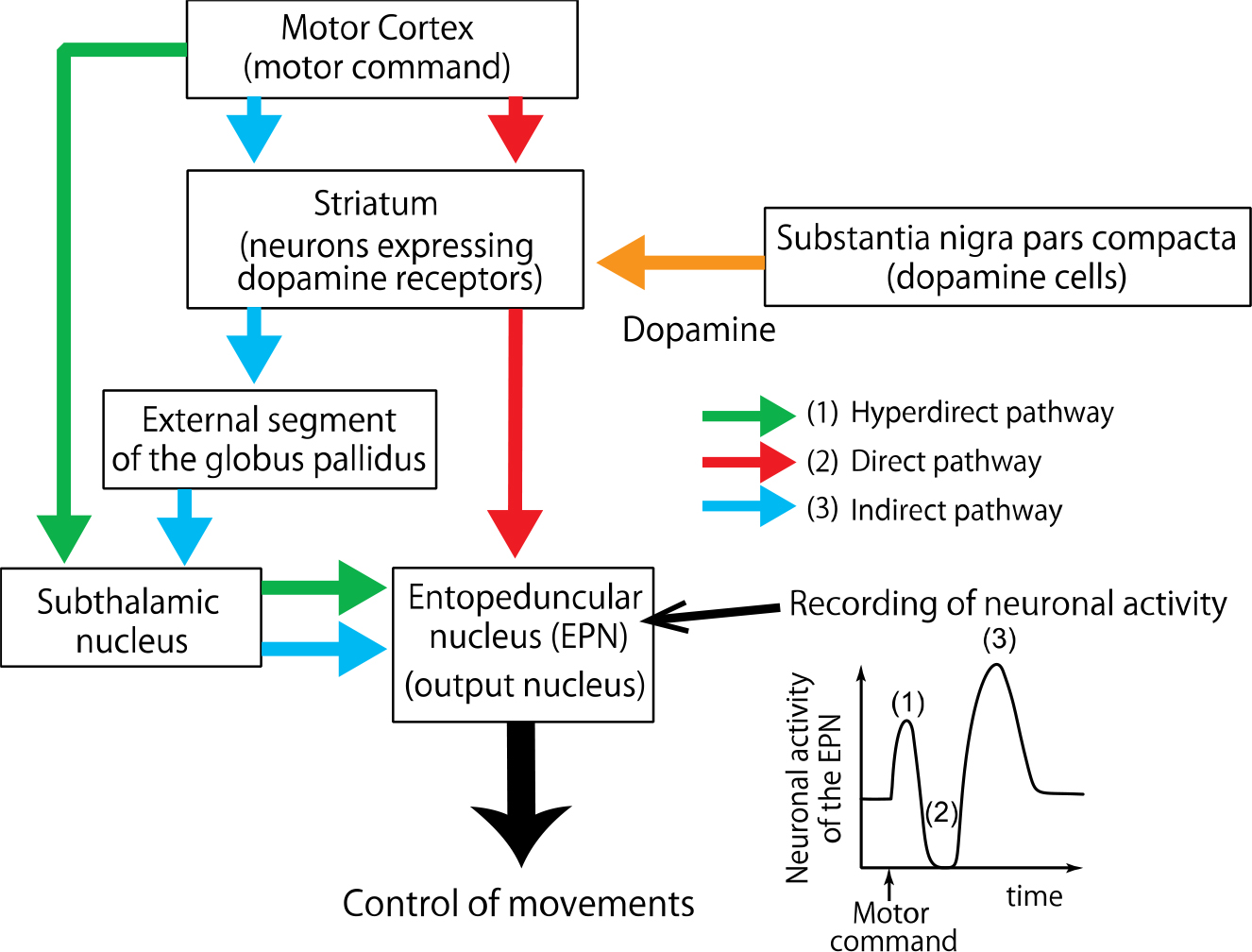
Electrical stimulation applied to the motor cortex induces neuronal activity in the basal ganglia that mimics information flow during voluntary movements. The stimulation induces triphasic response composed of early excitation, inhibition, and late excitation in neurons of the entopeduncular nucleus (EPN), the output station of the basal ganglia. Dopamine binds to dopamine receptors expressed in neurons of the striatum, and controls information flow through the three pathways in the basal ganglia.
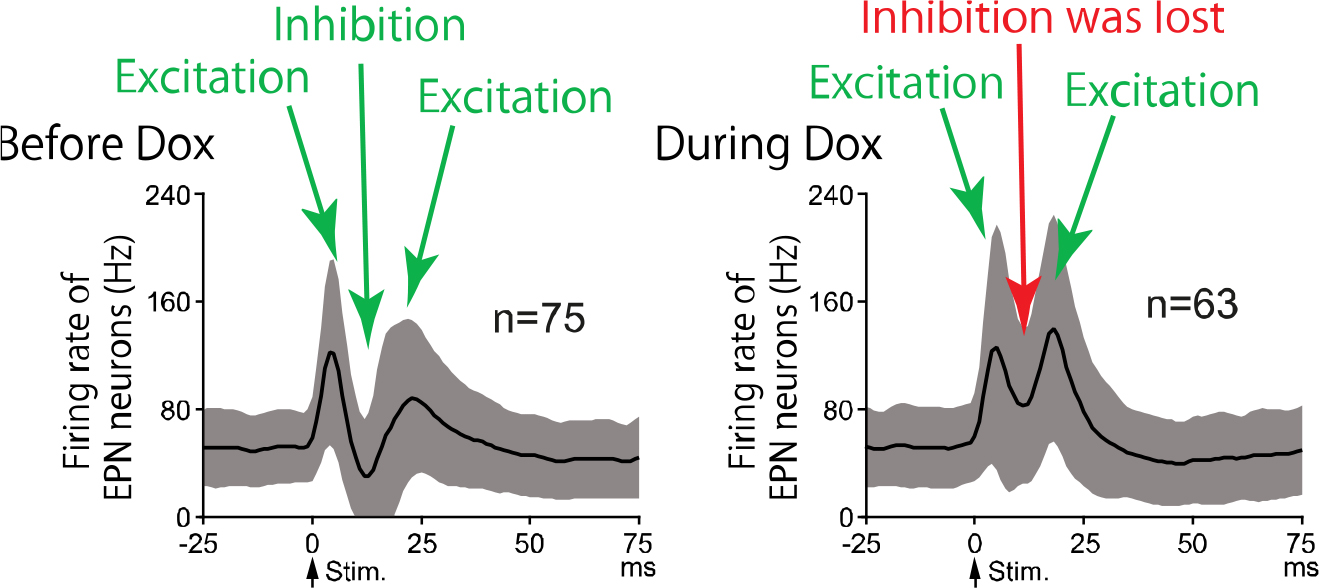
Neuronal activity of EPN neurons in response to electrical stimulation of the motor cortex was examined. Before Dox treatment, cortical stimulation induced trhiphasic response in EPN neurons (left). During Dox treatment (right), the inhibition that is mediated by the direct pathway was lost.
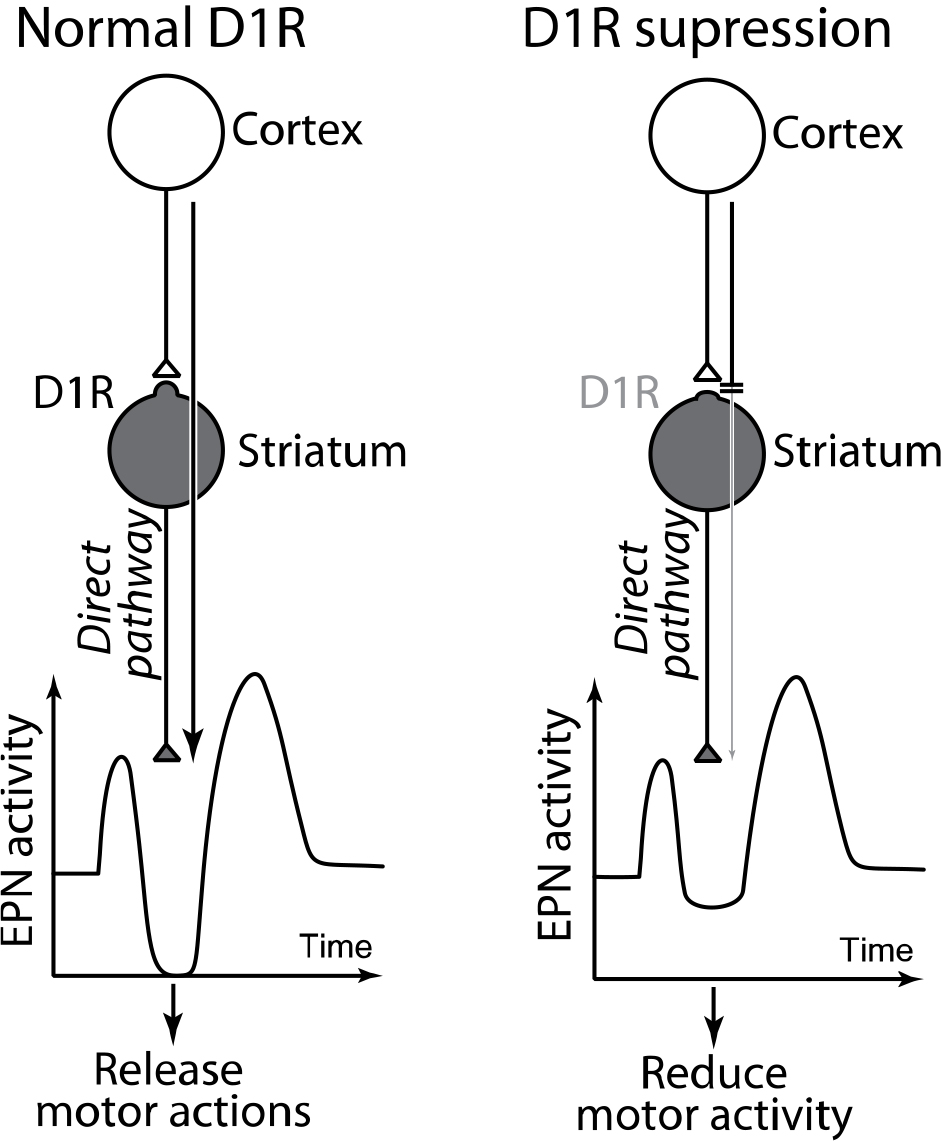
Under normal D1 receptor (D1R) expression (left), signals through the direct pathway induce inhibition in the EPN and release motor actions. During D1 receptor suppression (right), the information flow through the direct pathway is strongly suppressed and fails to induce inhibition in the EPN, which results in reduced motor activity.