Stroke is a leading cause of death and chronic disability in adults, causing a heavy social and economic burden worldwide. However, no treatments exist to restore the neuronal circuitry after a stroke. While most neurons are generated during embryonic brain development, new neurons continue to be produced in the ventricular-subventricular zone (V-SVZ) of the adult brain. In rodent olfaction, immature new neurons called neuroblasts form chain-like aggregates that migrate to the olfactory bulb, where they differentiate into interneurons. However, in the 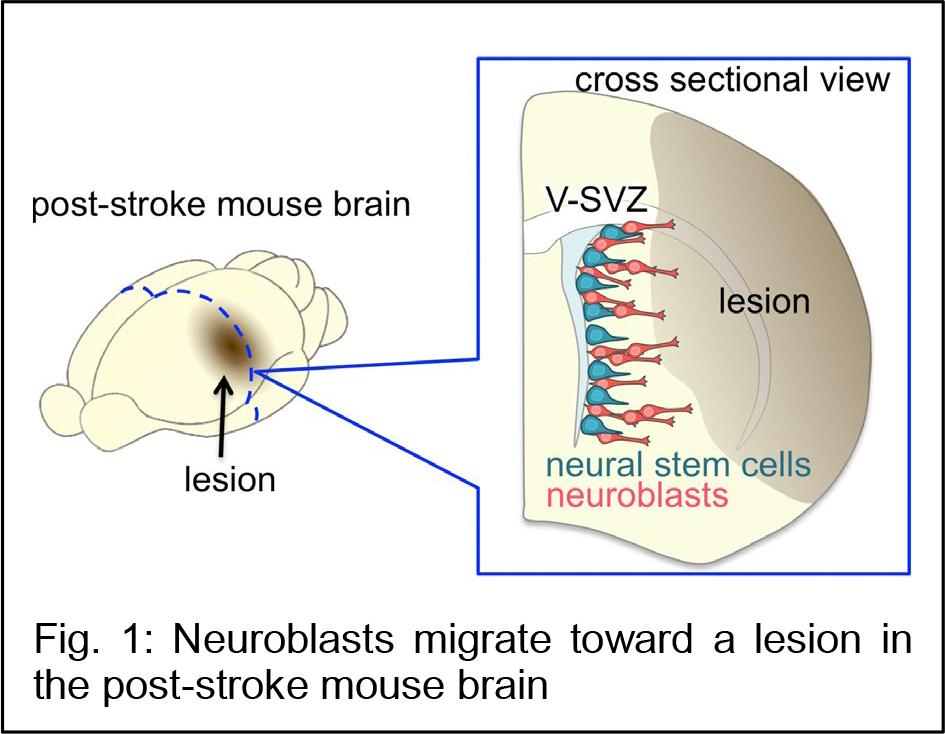 case of brain injury, the mammalian brain has only a limited ability to regenerate neuronal circuits for functional recovery. In a rodent ischemic stroke model induced by transiently blocking the middle cerebral artery, the most commonly affected vessel in human patients, some V-SVZ-derived neuroblasts migrate toward the lesion (Fig. 1), where they mature and become integrated into the neuronal circuitry. However, the number of these new neurons is insufficient to restore neuronal function.
case of brain injury, the mammalian brain has only a limited ability to regenerate neuronal circuits for functional recovery. In a rodent ischemic stroke model induced by transiently blocking the middle cerebral artery, the most commonly affected vessel in human patients, some V-SVZ-derived neuroblasts migrate toward the lesion (Fig. 1), where they mature and become integrated into the neuronal circuitry. However, the number of these new neurons is insufficient to restore neuronal function.
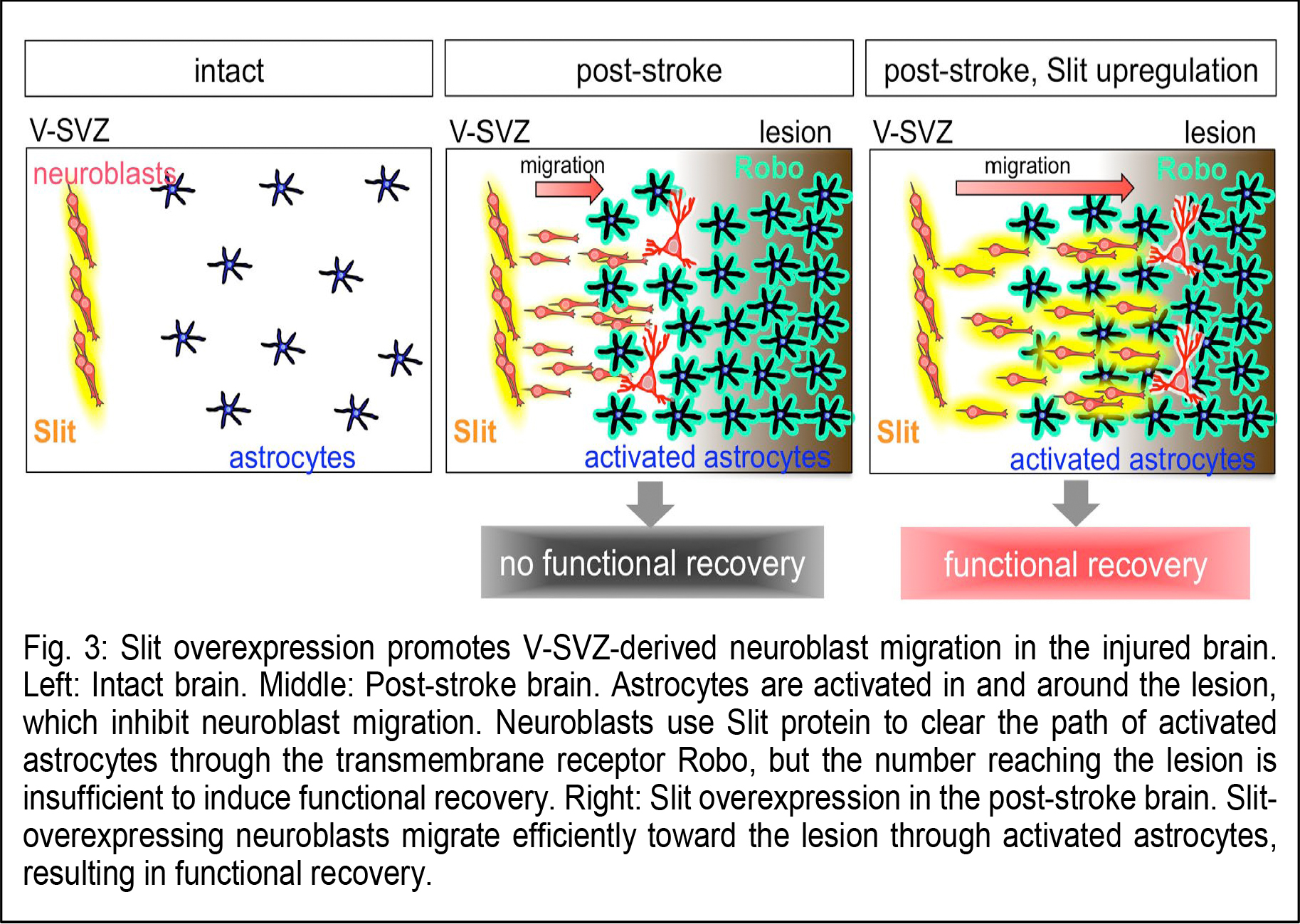
Dr. Kazunobu Sawamoto (Professor, Nagoya City University and NIPS) and Dr. Naoko Kaneko (Associate professor, Nagoya City University) in collaboration with Dr. Atsushi Nambu (Professor, NIPS) and Dr. Yasuo Kawaguchi (Professor, NIPS) have revealed a novel mechanism for neuronal regeneration, using the mouse model for ischemic stroke. Within a few days after stroke, astrocytes, a major population of macroglia, in and around the injured area become activated, exhibiting larger cell bodies, thicker processes, and proliferative behavior. The migrating neuroblasts must navigate through this astrocyte meshwork to reach the lesion. 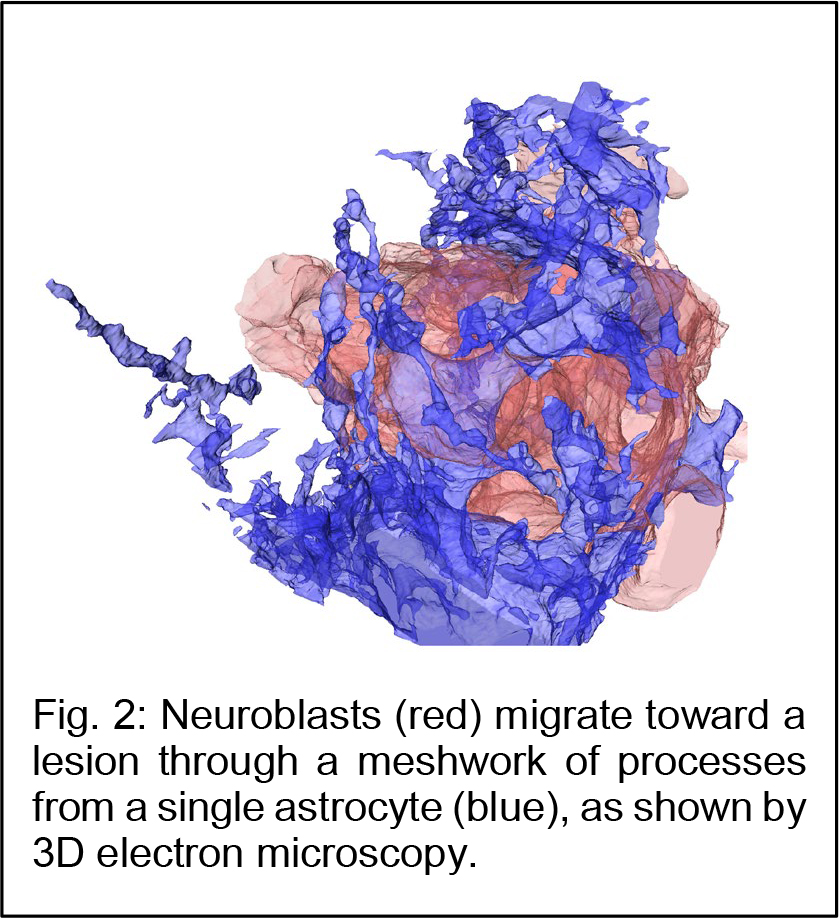 Using three-dimensional electron microscopy and live imaging, the research team demonstrated that neuroblast migration is restricted by the activated astrocytes in and around the lesion (Fig. 2). In normal, olfaction-related migration, neuroblasts secrete a protein called Slit, which binds to a receptor called Robo expressed on astrocytes. Slit alters the morphology of activated astrocytes at the site of neuroblast contact, to move the astrocyte surface away and clear the neuroblast’s migratory path. However, in the case of brain injury, the migrating neuroblasts actually down-regulated their Slit production, crippling their ability to reach the lesion for functional regeneration. Notably, overproducing Slit in the neuroblasts enabled them to migrate closer to the lesion, where they matured and regenerated neuronal circuits, leading to functional recovery in the post-stroke mice (Fig. 3). These results suggest that strategies designed to help migrating neurons reach the lesion may improve stem/progenitor cell-based therapies for brain injury.
Using three-dimensional electron microscopy and live imaging, the research team demonstrated that neuroblast migration is restricted by the activated astrocytes in and around the lesion (Fig. 2). In normal, olfaction-related migration, neuroblasts secrete a protein called Slit, which binds to a receptor called Robo expressed on astrocytes. Slit alters the morphology of activated astrocytes at the site of neuroblast contact, to move the astrocyte surface away and clear the neuroblast’s migratory path. However, in the case of brain injury, the migrating neuroblasts actually down-regulated their Slit production, crippling their ability to reach the lesion for functional regeneration. Notably, overproducing Slit in the neuroblasts enabled them to migrate closer to the lesion, where they matured and regenerated neuronal circuits, leading to functional recovery in the post-stroke mice (Fig. 3). These results suggest that strategies designed to help migrating neurons reach the lesion may improve stem/progenitor cell-based therapies for brain injury.
Journal: Science Advances
Title: New neurons use Slit-Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration
Authors: Naoko Kaneko1, Vicente Herranz-Pérez†2,3, Takeshi Otsuka†4,5, Hiromi Sano†5,6, Nobuhiko Ohno†7,8, Taichi Omata1, Huy Bang Nguyen8,9, Truc Quynh Thai8, Atsushi Nambu5,6, Yasuo Kawaguchi4,5, José Manuel García-Verdugo2, Kazunobu Sawamoto1,10*
Affiliations
1: Department of Developmental and Regenerative Biology, Nagoya City University Graduate School of Medical Sciences
2: Laboratory of Comparative Neurobiology, Instituto Cavanilles, Universidad de Valencia, CIBERNED
3: Predepartamental Unit of Medicine, Faculty of Health Sciences, Universitat Jaume I
4: Division of Cerebral Circuitry, National Institute for Physiological Sciences
5: Department of Physiological Sciences, SOKENDAI (The Graduate University for Advanced Studies)
6: Division of System Neurophysiology, National Institute for Physiological Sciences
7: Department of Anatomy, Division of Histology and Cell Biology, Jichi Medical University, School of Medicine
8: Division of Neurobiology and Bioinformatics, National Institute for Physiological Sciences
9: Department of Anatomy, Faculty of Medicine, University of Medicine and Pharmacy (UMP) at Ho Chi Minh city
10: Division of Neural Development and Regeneration, National Institute for Physiological Sciences
*Corresponding author
†These authors contributed equally to this work
Contact information
Department of Developmental and Regenerative Biology, Nagoya City University Graduate School of Medical Sciences
Address: 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
E-mail: sawamoto@med.nagoya-cu.ac.jp
Telephone: +81-52-853-8532
Fax: +81-52-851-1898
This work was supported by research grants from NEXT, MEXT, JSPS, the Brain Science Foundation, Grant-in-Aid for Research at Nagoya City University, the Takeda Science Foundation, and the Cooperative Study Programs of the National Institute for Physiological Sciences.


Nagoya City University
National Institute for Physiological Sciences (NIPS)