Studying molecular mechanisms of learning and memory is important to confront memory-deficient and abnormal memory-associated disorders such as post-traumatic stress disorder (PTSD). An enzyme called Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα ) that is abundant in the brain and phosphorylates important proteins has a key role in such mechanisms. CaMKIIα is especially enriched on the post-synaptic side of the synapse where neurons send messages from one to another, and is considered to be an indispensable molecule for long-term potentiation of the synapse (LTP), a fundamental mechanism for learning and memory. To explore its in vivo function more in detail and more explicitly, we generated the kinase-dead knock-in mutant mouse of CaMKIIα [CaMKIIα (K42R)-KI], and have been examining the mouse by using biochemical, electrophysiological, histological and behavioral methods in collaboration with other researchers. We are now exploring the relation between this kinase activity and age-related decline of cognitive functions by behavioral analyses of learning and memory.
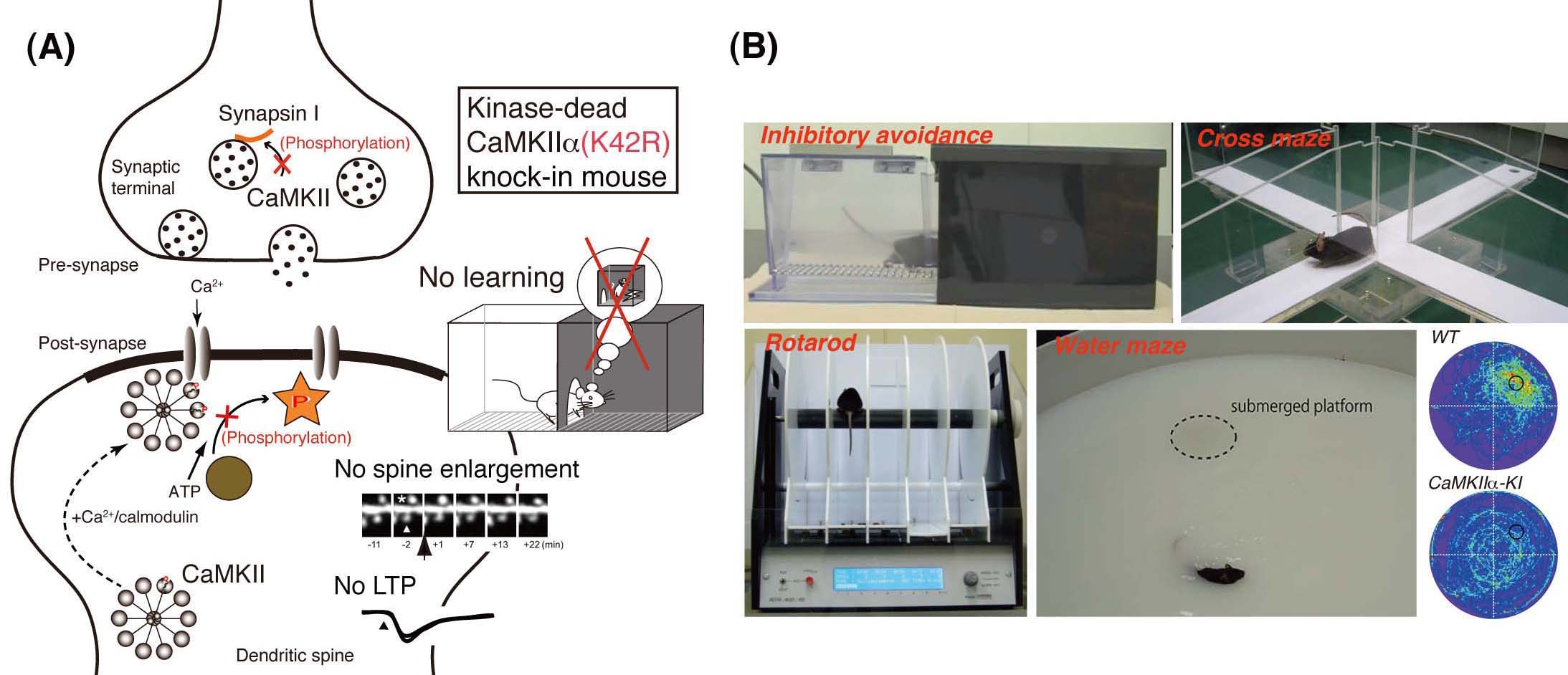 (A) In the kinase-dead CaMKIIα (K42R)-knock-in mouse (CaMKIIα-KI), long-term potentiation (LTP) of the hippocampal synapse and hippocampus-dependent learning and memory are severely impaired. (B) We are performing behavioral analyses to explore possible relationship between this kinase activity and age-related decline of cognitive functions.
(A) In the kinase-dead CaMKIIα (K42R)-knock-in mouse (CaMKIIα-KI), long-term potentiation (LTP) of the hippocampal synapse and hippocampus-dependent learning and memory are severely impaired. (B) We are performing behavioral analyses to explore possible relationship between this kinase activity and age-related decline of cognitive functions.
*Yamagata, Y. and Nairn, A.C. (2015) Contrasting features of ERK1/2 activity and synapsin I phosphorylation at the ERK1/2-dependent site in the rat brain in status epilepticus induced by kainic acid in vivo. Brain Res. 1625: 314-323.
doi: 10.1016/j.brainres.2015.08.023.
*Yamagata, Y., Kaneko, K., Kase, D., Ishihara, H., Nairn, A.C., Obata, K. and Imoto, K. (2013) Regulation of ERK1/2 mitogen-activated protein kinase by NMDA-receptor-induced seizure activity in cortical slices. Brain Res. 1507: 1-10.
doi: 10.1016/j.brainres.2013.02.015.
*Yamagata, Y., et al. (2009) Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIα in dendritic spine enlargement, long-term potentiation, and learning. J. Neurosci. 29: 7607-7618.
doi: 10.1523/JNEUROSCI.0707-09.2009.